
Childhood Immunization
Lec.5
Dr.Farah Sameer Yahya
16/10/2019
Immunisation :
is the process of protecting individuals from infection by inducing passive
or active immunity.
Passive immunity is induced by administering antibodies
Active immunity by stimulating the adaptive immune system using a vaccine.
Vaccination is one of the most important achievements in public health in the twentieth century.The most
salient feature of this achievement is decreased disease levels.The terms vaccine and vaccination originate
from the use of the cowpox virus Vaccinia (from latin vacca = cow) to protect against smallpox.
After access to clean drinking-water, immunisation is the most effective public health measure for
promoting health and has saved millions of lives.
Vaccination led to the eradication of smallpox in 1980 and a similar program has almost eradicated polio.
What is the difference between an immunization and a vaccination
Although the two words are often used interchangeably, the CDC defines the two as follows:
Vaccination: The act of introducing a vaccine into the body to produce immunity to a specific
disease.
Immunization: A process by which a person becomes protected against a disease through
vaccination. This term is often used interchangeably with vaccination or inoculation.
Active immunization relies on stimulating the immune system with a vaccine which mimics the pathogen
but does not cause the disease.The adaptive immune system has a memory so that immunity is generated
to specific pathogens that a host has encountered.
Routine childhood vaccination is one of the few health care interventions that saves both lives and dollars;
compared with a no vaccination program.
TYPES OF Vaccines:
Currently available vaccines are either live or inactivated.
Live vaccines include:
Those that protect against viruses (eg, MMR, varicella, rotavirus, live attenuated influenza intranasal
vaccine [LAIV], yellow fever, vaccinia, oral polio vaccine)

Those that protect against bacteria (eg, BCG, oral typhoid vaccine).
1. Live vaccines replicate in the body.
2. Live injected vaccines usually induce immunity through a single dose
3. and unlike inactivated vaccines, are susceptible to vaccine failure caused by circulating antibodies,
including residual maternal antibodies in infants.
Inactivated vaccines include:
Inactivated viruses
(eg, inactivated polio vaccine [IPV], hepatitis A, rabies)
Inactivated subunits
(eg, acellular pertussis, hepatitis B, human papillomavirus [HPV], split virus influenza, typhoid Vi)
Toxoid (diphtheria, tetanus) agents
Polysaccharides either unconjugated (pneumococcal, meningococcal) or conjugated (Haemophilus
influenzae type b [Hib], pneumococcal, meningococcal)
1. Inactivated vaccines do not contain infectious particles that can replicate in the body;
2. They generally require several doses to immunize the patients completely.
Contraindications to immunisation
1. A confirmed anaphylactic reaction to a previous dose of a vaccine containing the same antigens or
another component of the vaccine e.g. neomycin
2. Live vaccines: may be temporarily contraindicated in individuals who are immunosuppressed or
pregnant.
3. Egg allergy: a previous anaphylactic reaction to egg contraindicates influenza and yellow fever
vaccines.
MMR vaccine can be given safely to most children with egg allergy, but should be done under controlled
conditions in those with a history of anaphylaxis.
4. Acute illness with fever or systemic upset: immunization should be postponed until recovery. Minor
illness does not require postponement.
5. Children with an evolving neurological condition: immunization should be deferred until the
condition has resolved or stabilized and a diagnosis has been made.
False ‘contraindications’
The following are not contraindications to immunisation:
1. Personal or family history of : seizures (febrile or afebrile) with no evidence of neurological
deterioration
2. autistic spectrum disoroders
3. Asthma or hay fever
4. Pregnancy of child”s mother
5. Being under certain surgery
6. Previous history of measles or rubella
7. Stable neurological conditions e.g cerebral palsy
Live vaccines and immunosuppression
Live vaccines such as BCG and MMR are contraindicated in the following infants and children with defective
immunity:
1. Children treated for malignant disease with chemotherapy or radiotherapy either currently or within
the last 6 months.
2. Transplant patients: all solid organ transplant patients on immunosuppressant drugs or patients
who have had a bone marrow transplant until 12 months after finishing immunosuppressive
treatment.
3. Children who have received high-dose steroid treatment within the previous 3 months.
Or Children who have received immunosuppressant drugs (e.g. azathioprine, methotrexate)
4. Patients with severe primary immunodeficiency,or Children with HIV infection .
HIV-positive children can receive MMR unless there is severe immunosuppression when measles
vaccine is a risk.
They should not receive BCG, yellow fever, or oral typhoid vaccines.
Inactivated polio virus vaccination is preferable.
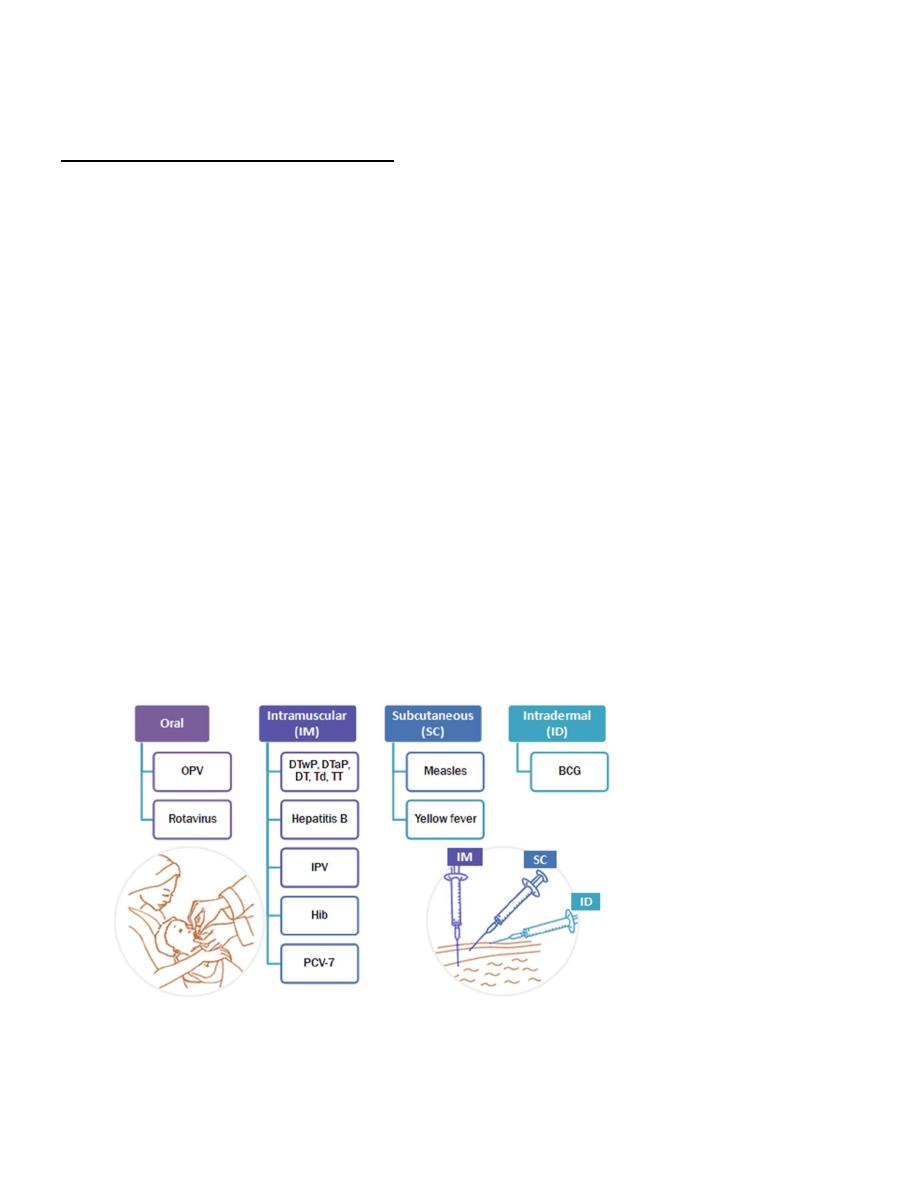
Route of administration of vaccines
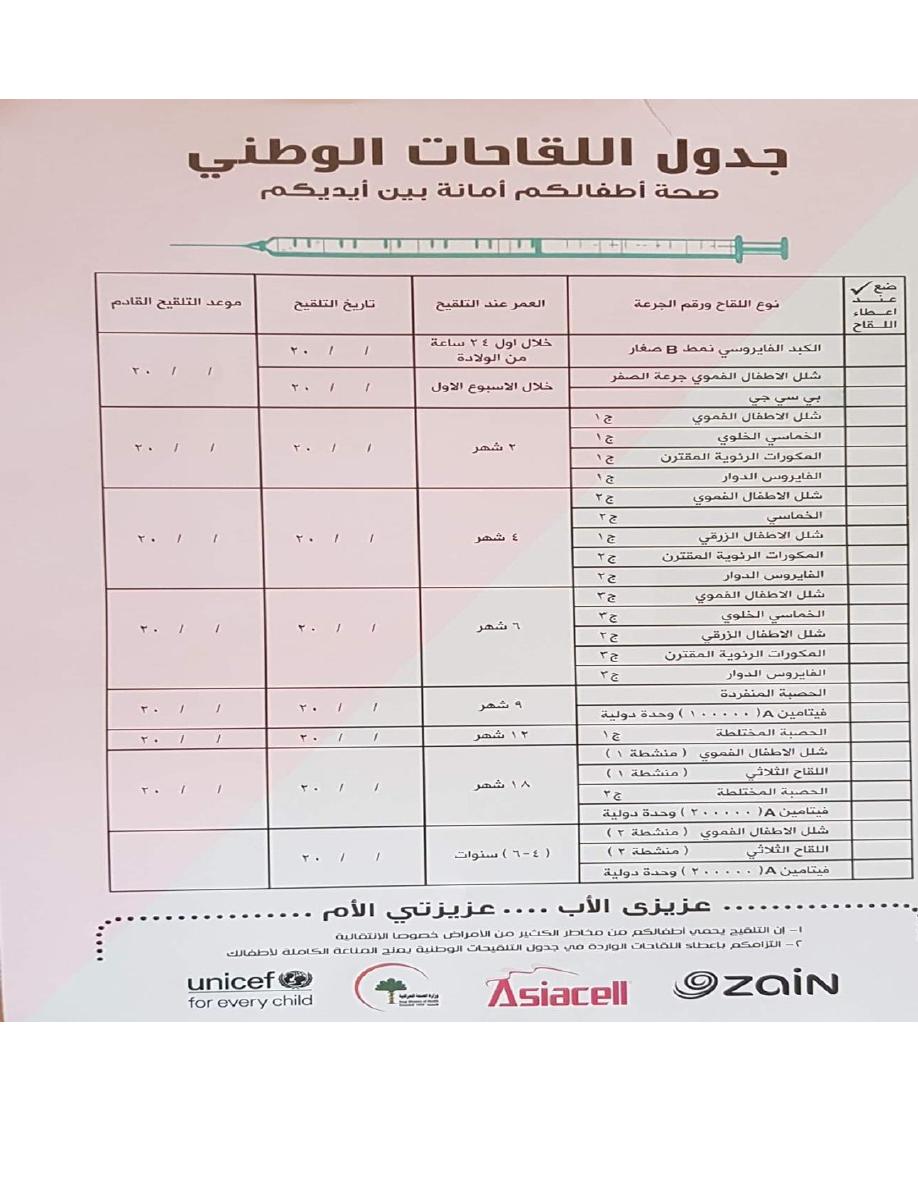
THE IRAQI NATIONAL IMMUNIZATION PROGRAMM

DaPT ( Diphtheria-Tetanus-Acellular Pertussis Vaccine)
Diphtheria is caused by infection with Corynebacterium diphtheria in the pharynx or skin. Diphtheria
vaccine: D,d
The vaccine is a cell-free purified toxin treated with formaldehyde to form toxoid and absorbed on to an
adjuvant. It is produced in two strengths: D (30 iu) and d (2 iu). The low dose of vaccine is used for primary
immunisations in children >10 years and in the booster at 13–15 years.
Diphtheria vaccine is available only in combined products.
Tetanus
Tetanus is an acute disease caused by tetanus toxin released following infection by Clostridium tetani , the
spores of which are present in soil or manure. Maternal and neonatal tetanus due to infection of the
umbilical stump remains an important cause of death in many countries.
Tetanus vaccine: T
Tetanus vaccine is a cell-free purified toxin treated with formaldehyde to form toxoid and absorbed on to
an adjuvant. It is given only as part of combined products.
Pertussis
Pertussis (whooping cough) is caused by infection with Bordetella pertussis, or less commonly by B.
parapertussis . Severe complications such as bronchopneumonia and cerebral hypoxia or death are most
common in infants <6 months.
Pertussis vaccine: aP Whole-cell pertussis vaccines have been replaced by acellular vaccines, aP, made from
highly purified selected components of the organism which are treated with formaldehyde and absorbed
on to adjuvants. The pertussis vaccines are given only as part of combined products.
ADMINISTRATION The vaccine is administered intramuscularly
ADVERSE REACTIONS. Adverse reactions to diphtheria and tetanus toxoids include (1) local reactions, such
as redness and induration, (2) nodule at the injection site, and (3) Arthus-type hypersensitivity reactions.
Moderate-to-severe reactions include high fevers of 40.5° C or higher, persistent and inconsolable crying of
more than 3 hours' duration, hypotonic-hyporesponsive episodes, and febrile seizures. All of these
reactions occur much less frequently with DTaP than with DTP, and all are believed to occur without
permanent sequelae.
CONTRAINDICATIONS AND PRECAUTIONS
Contraindication to DTaP vaccination include:-
(1) a history of severe (anaphylactic) allergic reaction after a prior dose of DTaP vaccine or a vaccine
component and

(2) encephalopathy within 7 days of a previous dose.
Precautions to DTaP :include
1. a convulsion within 3 days of a previous dose;
2. persistent, severe, inconsolable screaming or crying for 3 or more hours within 48 hours of a
previous dose;
3. collapse or shock like state within 48 hours of a previous dose;
4. and a temperature of 40.5° C (105° F) that is unexplained by another cause within 48 hours of a
previous dose.
5. Vaccination should be deferred in the event of a moderate to severe acute illness until the illness
subsides.
POLIO VACCINES
The availability of two effective vaccines against poliomyelitis for the past five decades has ensured
remarkable decline in the global burden of disease. They were developed in the USA during 1950s, first the
inactivated polio vaccine (IPV) by Jonas Salk and later the live oral polio vaccine (OPV) by Albert Sabin.
The Global Polio Eradication initiative was launched in 1988 using oral polio vaccine (OPV) as the
eradication tool.
OPV
OPV is a trivalent vaccine consisting of a suspension of attenuated poliovirus types1, 2 and 3 .
When OPV is given by mouth, the vaccine viruses reach the intestines where they must establish infection
(vaccine virus “take”) before an immune response may occur.
The onset of action of OPV is faster as compared to inactivated poliovirus vaccine (IPV) and thus OPV is the
vaccine of choice for outbreak control.
A rare but serious adverse effect associated with OPV is vaccine associated paralytic poliomyelitis
(VAPP).
VAPP occurs due to loss of attenuating mutations and reversion to neurovirulence during replication
of the vaccine virus in the gut.
VAPP is defined as those cases of AFP which have residual weakness 60 days after the onset of
paralysis and from whose stool samples, vaccine-related poliovirus but no wild polio virus is isolated.
VAPP may occur in the vaccine recipient or contact of the vaccine recipient (contact VAPP).
The risk of VAPP is higher with the first dose that “takes”, with P2 virus and in patients with B cell
immunodeficiency.
The incidence of VAPP has been estimated at 4 cases per million (1/1 000 000) birth cohort per year
in countries using OPV.
OPV is contraindicated in immunodeficient patients (especially humoral immunodeficiencies) and
their household contacts.

Inactivated polio vaccines (IPV)
IPV is killed poliovirus.
It is highly immunogenic. Its immunogenicity is dampened by the presence of maternal antibody in the
very young infant, especially up to the age of 8 weeks.
Several countries have shifted from all OPV to sequential OPV- IPV schedules and all IPV schedules with
elimination of wild polio. IPV will be indispensable in the post eradication era when OPV has to stop but
“vaccination against polio” cannot stop.
It was widely believed that OPV mimics natural infection, spreads widely in the community and immunizes
children in community (contact immunization), and produces excellent mucosal immunity to protect not
only from disease but also from subsequent infection.
Due to these attributes, it was believed that it would eliminate transmission of wild polio virus when 3
dose OV coverage reaches 80-85% (Herd Effect).
0
Pneumococcal vaccine 13-valent (PCV)
(PREVNAR 13)
The 13 valent pneumococcal conjugate vaccine indicated for active immunization to prevent invasive
disease caused by S pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F
Routine vaccination recommended for all infants in Iraq 3 dose series at ages 2, 4, and 6 months.
For children age 6 through 18 years of age who previously have not received PCV13 OR PPSV23
(pneumoccocal polysaccharide vaccine 23) who at increase risk of invasive pneumococcal infection
due to anatomical or functional asplenia including sickle cell disease HIV infection choclear implant
CSF leak or other immunocompromizing conditions(e.g nephrotic syndrome ) administration of a
single PCV 13 dose followed by a dose of PPSV23 dose at least 8wks apart .
Haemophilus influenzae type b (Hib)
Haemophilus influenzae can cause serious invasive disease, especially in young children. Invasive
disease is usually caused by encapsulated strains Type b was the most prevalent strain.
Invasive Hib diseases include meningitis, septicaemia, septic arthritis, osteomyelitis, and pneumonia.
Development of highly effective conjugate Hib vaccines, first licensed in 1987.
Rotavirus vaccine
Rotavirus is the most common cause of severe gastroenteritis in infants and young children.
A trivalent rotavirus vaccine was licensed in the USA in 1998 and was subsequently linked to an
increased risk for intussusception, The vaccine was withdrawn from the market in 1999. Subsequently

2 new live, oral rotavirus vaccines have been approved in the USA after extensive safety and efficacy
testing.
Rotarix
is a live, monovalent, human attenuated vaccine given in 2 doses at 2 and 4 months of age.
RotaTeq
is a live, pentavalent, human–bovine reassortant vaccine given in 3 doses at 2, 4 and 6
months of age.
Rotavirus vaccines.(RotaTeq) (minimum age: 6 weeks)
it is given 3-dose orally series at 2, 4, and 6 months
Do not start the series on or after age 15 weeks, 0 days.
The maximum age for the final dose is 8 months, 0 days.
Rotarix new monovalent rotavirus vaccine was licensed in the USA (now it is used in Iraqi national
program od vaccination) and also appears to be safe and effective. It is an attenuated monovalent
human rotavirus and is administered as 2 oral doses at 2 and 4 mo of age. The vaccine has 85% efficacy
against severe gastroenteritis and was found to reduce hospital admissions for all diarrhea by 42%.
Despite being monovalent, the vaccine is effective in prevention of all 4 common serotypes of human
rotavirus.
BCG (Bacille Calmette-Guérin
Vaccination)
It is a live attenuated vaccine CONTAIN strain of M. bovis attenuated by sub –culture.
The BCG vaccines are extremely safe in immunocompetent hosts. Local ulceration and regional
suppurative adenitis occur in 0.1-1% of vaccine recipients. Most reactions are mild and usually resolve
spontaneously, but AB is needed occasionally. Surgical excision of a suppurative draining node is
rarely necessary and should be avoided if possible. Osteitis is a rare complication of BCG
vaccination.
BCG is 50% effective in preventing pulmonary tuberculosis in adults and children. The
protective effect for disseminated and meningeal tuberculosis appears to be slightly higher,
with BCG preventing 50-80% of cases.
recommendation of the World Health Organization is a single dose administered during
infancy, in populations where the risk for tuberculosis is high
BCG vacine is contraindicated in immunocompromised patient .
BCG is given as a single intradermal
injection
at the
insertion
of the
deltoid.

Side effects of BCG VACCINE
Very common side effect (affecting 9 out of 10 people): Hardness at the injection site, followed
by a raised blister.
Uncommon side effects (affecting up to 1 in 100 people at each dose):
1. Headache and a raised temperature (fever)
2. An ulcer which develops from the blister at the injection site, two to six weeks after injection.
This may be painful and take several weeks or months to heal fully.
3. Swelling of lymph nodes in the axilla larger than 1 cm across
4. An enlarged lymph node that becomes infected (lymphadenitis)
Rare side effects (affecting up to 1 in 1000 people at each dose):
More severe skin reactions. These usually heal within a few weeks.
Bone inflammation (osteitis or osteomyelitis)
An abscess at the injection site
MEASLES VACCINE and MMR
Vaccination against measles is the most effective and safe prevention strategy. In Iraqi national vaccination
program it is given at age 9 months then it is given in combination (MMR) with mump and rubella at age 12
months and then at 18 months in combination with vit.A
adverse events from the measles-mumps-rubella vaccine include fever (usually 6-12 days following
vaccination), rash in approximately 5% of vaccinated persons, and, rarely, transient thrombocytopenia.
Children prone to febrile seizures may experience an event following vaccination, so the risks and benefits
of vaccination should be discussed with parents. Encephalopathy andautism have not been shown to be
causally associated with the measles-mumps rubella vaccine or vaccine constituents.
A review of the effect of measles vaccination on the epidemiology of SSPE (subacute sclerosing
panencephalitis) has demonstrated that measles vaccination protects against SSPE and does not accelerate
the course of SSPE or trigger the disease in those already infected with wild measles virus.Passively
administered immune globulin may inhibit the immune response to live measles vaccine, and
administration should be delayed for variable amounts of time based on the dose of Ig.
OPV شلل االطفال الفموي
HBV الكبد الفايروسي,PENTA VACCINE يولخلا يسامخلا ,,ROTA VACCINE راودلا سوريافلا
IPV شلل االطفال الزرقي,,,,PCV نرتقملا ةيوئرلا تاروكملا,,,MEASLES VACCINE ةدرفنملا ةبصحلا
MMRالحصبة المختلطه…..DPT يثلاثلا حاقللا

