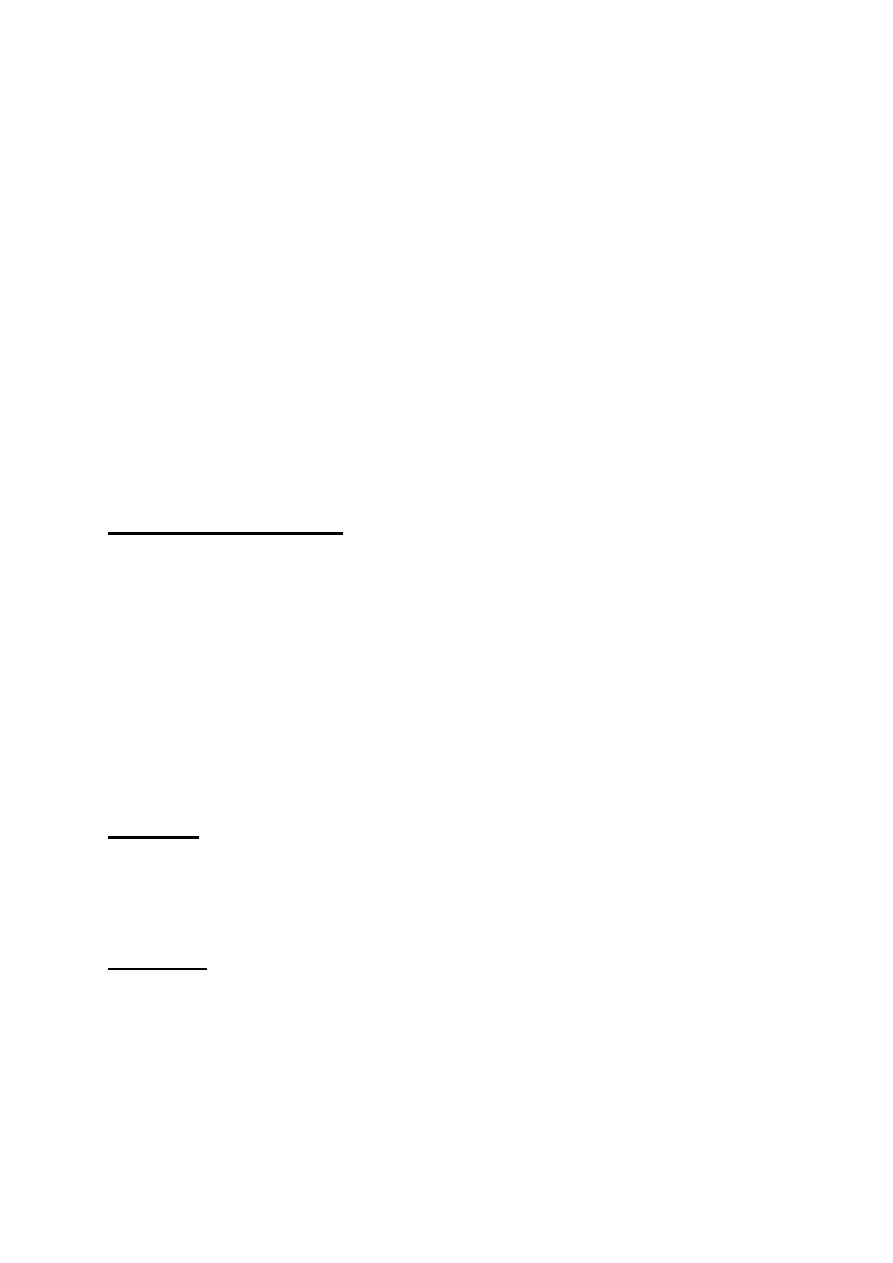
1
) ﻋﺪد اﻻوراق
14
(
ﻋﯿﻮن
2
2
/
1
1
/
2019
.د
ﻋﺰام
Lec: 9
Retina
Out-lines:
1- Anatomy and physiology of retina.
2- Retinitis pigmentosa
3- Age –related macular degeneration.
4- Retino-vascular disorders.
5- Toxoplasmosis.
6- Retinal tumours- retinoblastoma.
Anatomy and physiology:
Anatomical landmarks:
The macula is a round area at the posterior pole, lying inside temporal
vascular arcades, it measures 5-6 mm in diameter, and sub-serves the
central 15-20° of visual field. Histologically, it shows more than one
layer of ganglion cells, in contract to single cell layer of the peripheral
retina.
The inner layers of the macula contains the yellow xanthophyll
carotenoid pigments lutein and zeaxanthin in far higher
concentrations than peripheral retina.
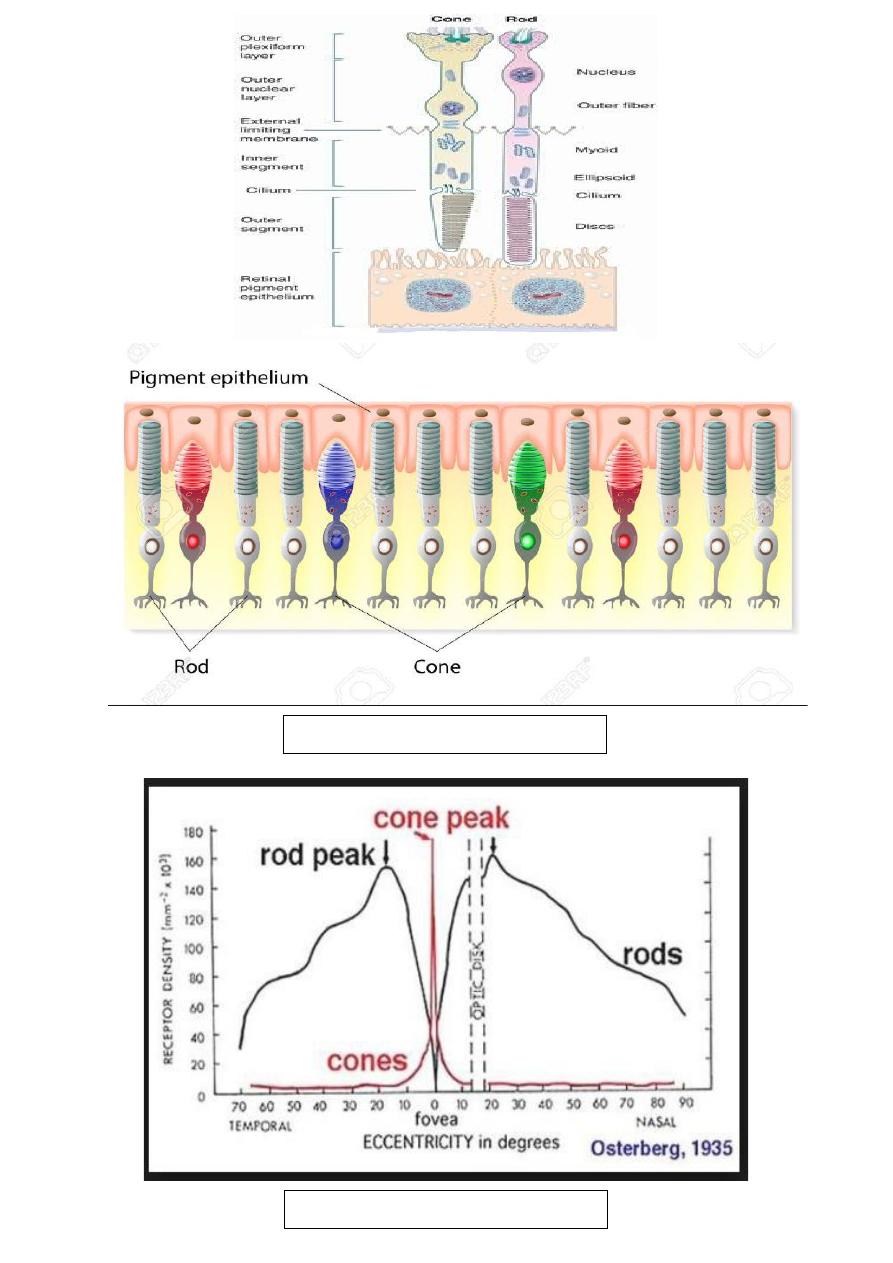
The rods: are most numerous (120 million), and are of the highest
concentration in the mid-peripheral retina. They are most sensitive in
dim illumination and they are responsible for night and peripheral
vision.
The cons: are fewer in number (6 million) and have their highest
concentration at the fovea. They are most sensitive in bright light and
mediate day vision, color vision and central visual acuity. Cone
dysfunction therefore results in poor central vision, impairment of
color vision (Dyschromatopsia) and occasionally problems with day
vision (hemeralopia).
2
Rods / cons distribution
Rods and cons
3
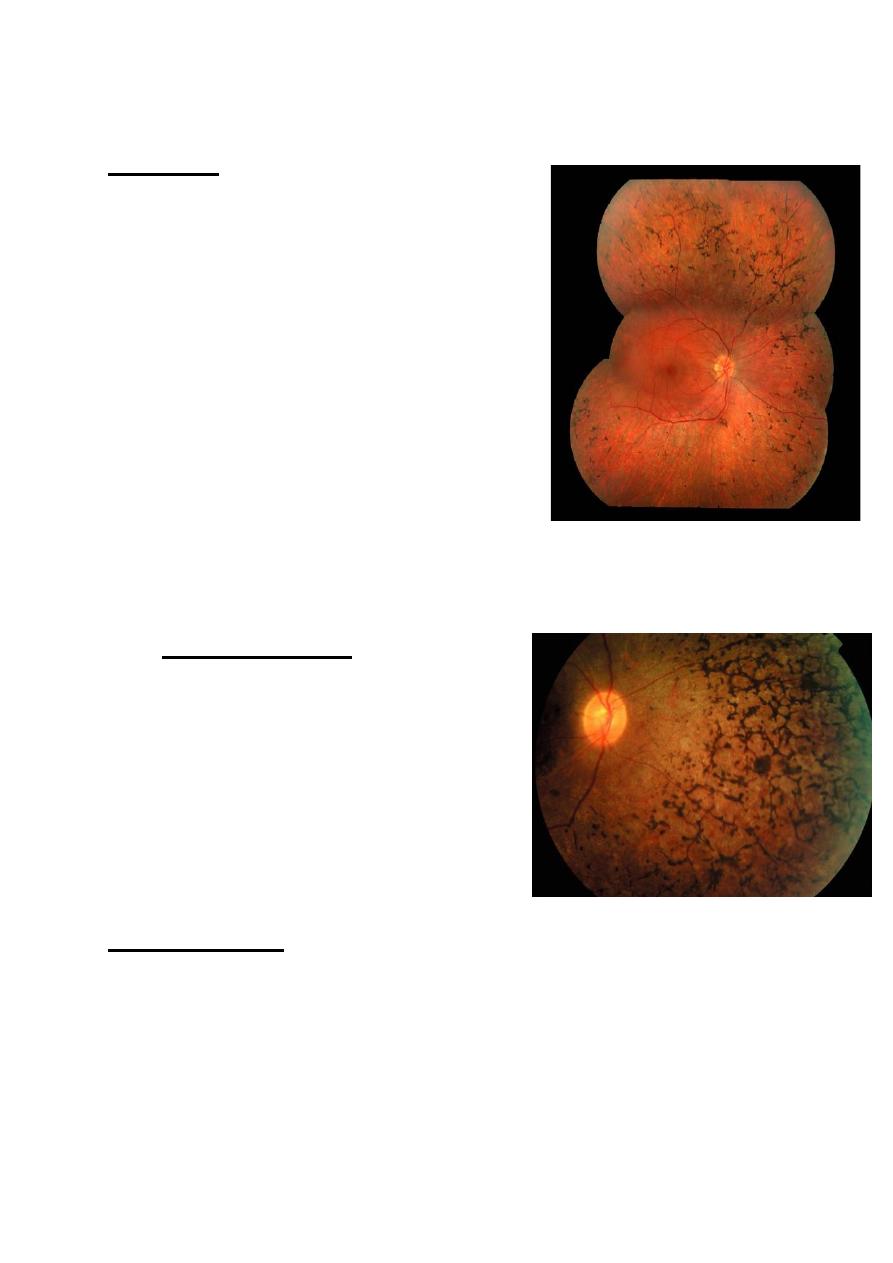
Retinitis pigmentosa:
Definition:
• It is a hereditary primary pigmentary
retinal dystrophy affecting the rods
more than cons , characterized by
night blindness associated with
typical pigmentary changes of fundus.
• Presentation of this uncommon
condition is during the second decade
of life with night blindness.
Inheritance may be autosomal
dominant, recessive or X-linked. A
large number of genetic abnormalities
have founded to cause the clinical picture, the hallmarks of
which are mid peripheral perivascular “bone-spicule”
pigmentation, arteriolar attenuation and waxy disc pallor.
• Ocular association
1 myopia.
2 POAG
3 microphthalmos.
4 keratoconus.
5 posterior subcapsular cataract.
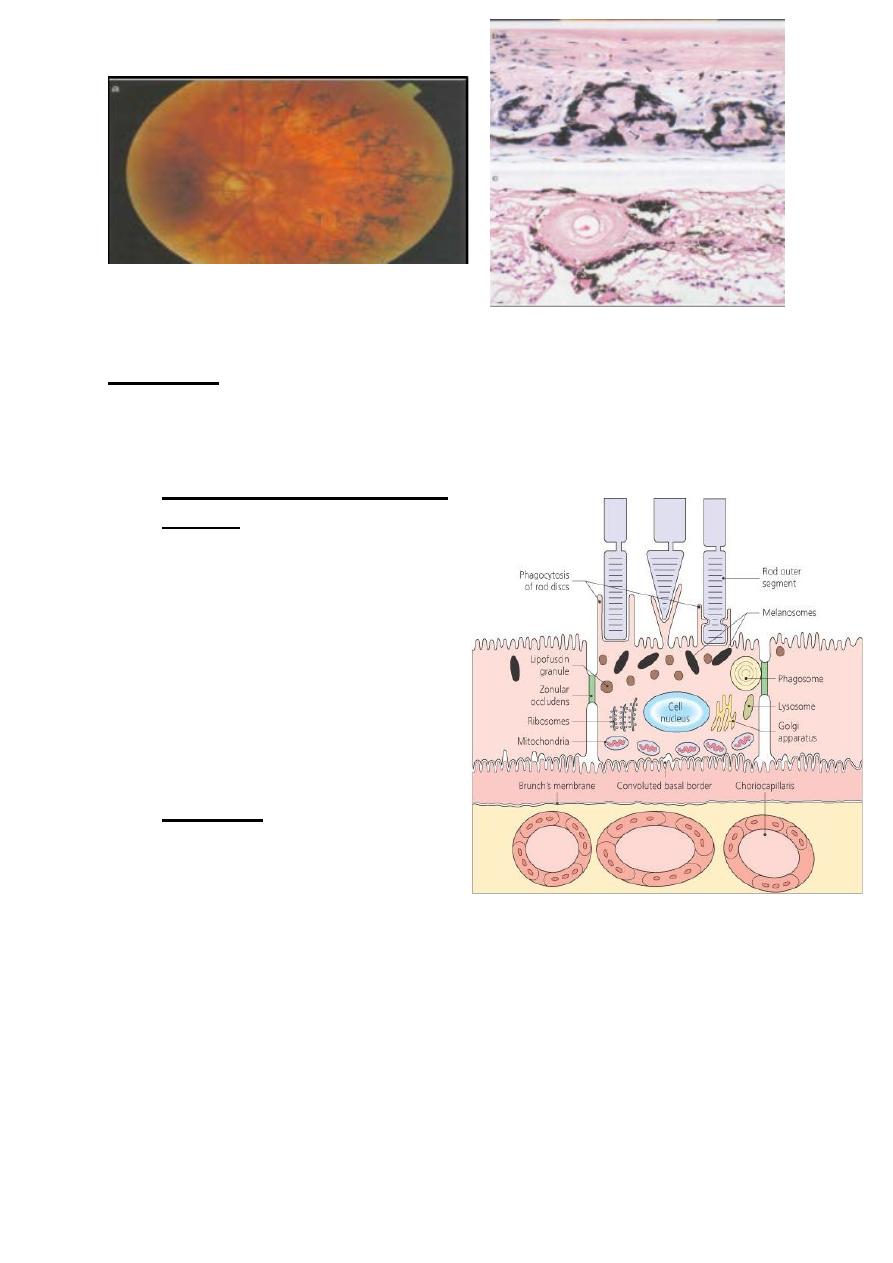
Histopathology: Photoreceptor degeneration, the final pathway
involves apoptosis of rod photoreceptors with cone degeneration at
later stage.
The RPE responds to photoreceptor atrophy by proliferation into the
retina. The pigmented cells accumulate around atrophic retinal
blood vessels, which explains the classical “ bone- spicule” fundus
appearance
4
Age- related macular degeneration (AMD):
Definition:
• It refers to degenerative changes seen in macular area in old
age. It’s the leading cause of blindness in developed countries.
• Retinal Pigment Epithelium
(RPE) : is composed of single
layer of cells, hexagonal in
cross-section.
• The cell base in contact with
Bruch’s membrane, and at the
cell apices multiple thread-
like villous process extend
between the outer segments of
photoreceptors.
• Functions: RPE cells and
intervening tight junction
complexes (zonula
occludents) constitute the
outer blood-retinal barrier, preventing extracellular fluid leaking
into sub retinal space from choriocapillaris, and actively pump
ions and water out of the sub-retinal space.
5
Age-related macular degeneration AMD:
• It is the most common cause of legal blindness in industrialized
societies. Patients are typically over the age of 65 years; both
eyes are usually affected, frequently asymmetrical.
• Clinical features;
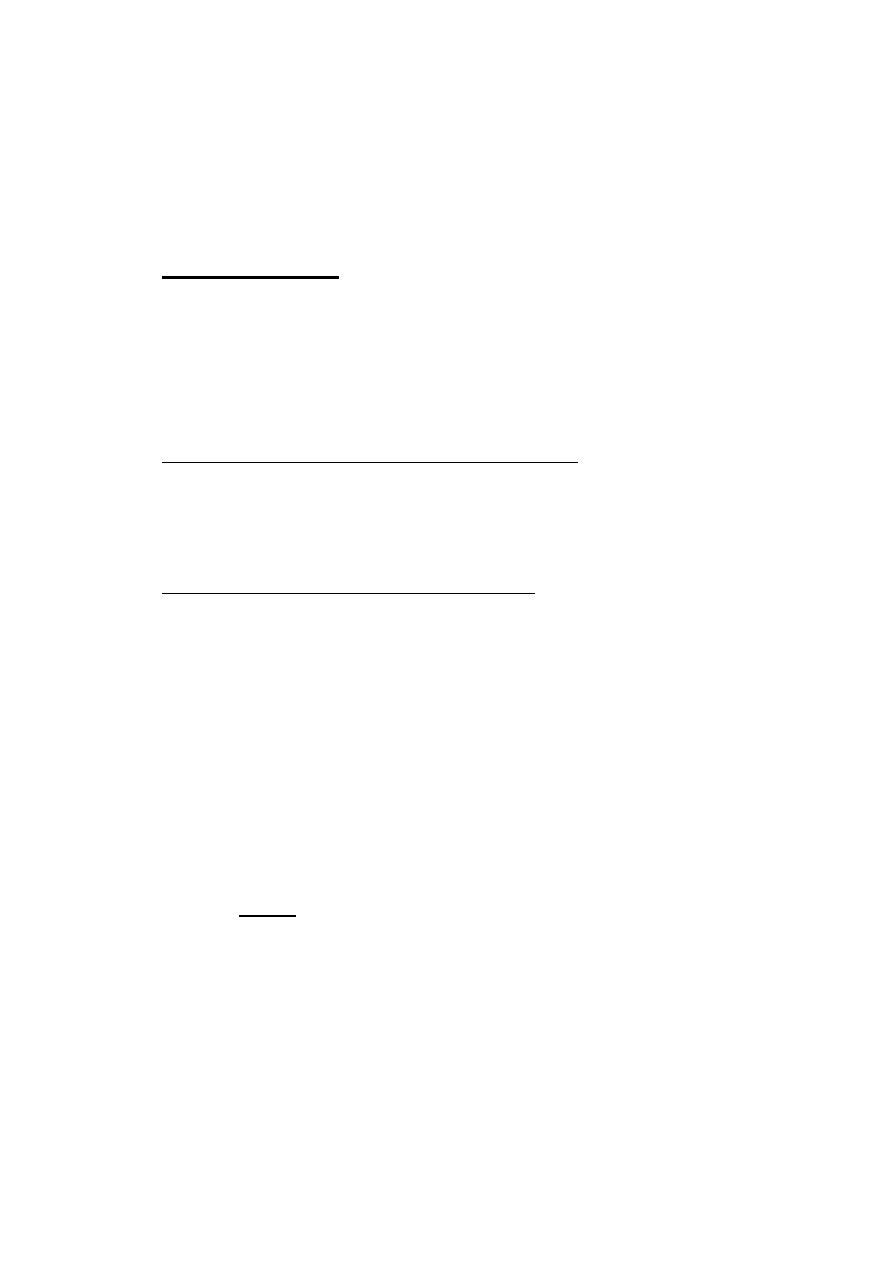
• 1-Hard drusen small, round, discrete, yellow-white lesions,
usually located at the macula.
• 2-Soft drusen larger lesions with ill-defined edges associated
with exudative AMD.
• Non-exudative (dry) macular degeneration atrophic and
hyperplasic changes of retinal pigment epithelium (RPE)
associated with slowly progressive degeneration of overlying
nueroretina ad underlying choriocapillaris.
• Exudative (wet) macular degeneration the ingrowth of choroidal
new vessels through Bruch’s membrane: choroidal neovascular
membrane (CNV). Presents with unilateral distortion of central
vision. On examination, an area of macula is elevated by sub-
retinal fluid and blood, often with associated clumps of
exudates. The lesion evolves in most cases to leave sub-retinal
“disciform scarring” with permanent loss of central vision.
• RPE detachment (PED) fluid elevates the RPE in a dome
configuration. This may progress to exudative AMD described
above.
Diagnosis of AMD follows well-known key steps. Most patients with
AMD present only after they have experienced some visual
disturbances, such as metamorphopsia, central scotoma or loss of
vision. Diagnosis may therefore occur when the condition is relatively
advanced and both eyes are affected. Visual acuity is measured using
charts such as the Snellen chart but grading varies between countries.
For consistency, the US grading system, with 20/20 for excellent
vision, will be used here to describe visual acuity.

6
Assessment of components
of CNV lesion secondary
to AMD
After other possible causes of vision loss, such as cataract, are
excluded, the pupil is dilated and biomicroscopy of the retina is
performed, using a slit lamp and a microscope, to visualize the macula
stereoscopically. Drusen can be seen as yellow spots within the
macular area in AMD.
If biomicroscopy suggests the development of neovascular AMD, for
example by the appearance of macular oedema or an elevated RPE,
the macula may be evaluated with fluorescein angiography to confirm
the presence of CNV, and its size, location, and other features which
may influence its management. Some centres in the TAP trial have
used indocyanine green (ICG) angiography as an ancillary study
modality.

7
Management:
• 1- Often of negligible or temporary benefit and therefore only
used in selected cases.
• 2- Antioxidant vitamin and mineral supplements may retard the
progression of AMD.
• 3-Conventional laser may be effective at destroying CNV that
does not encroach on central macula.
• 4- Photodynamic therapy (PDT) is a newer technique-using
laser to activate a light- sensitive dye preferentially taken up by
CNV.
• 5- Intravitreal injection of anti-vascular endothelial growth
factors (Anti-GEGFs) e.g. ranizumab and bevazumab.
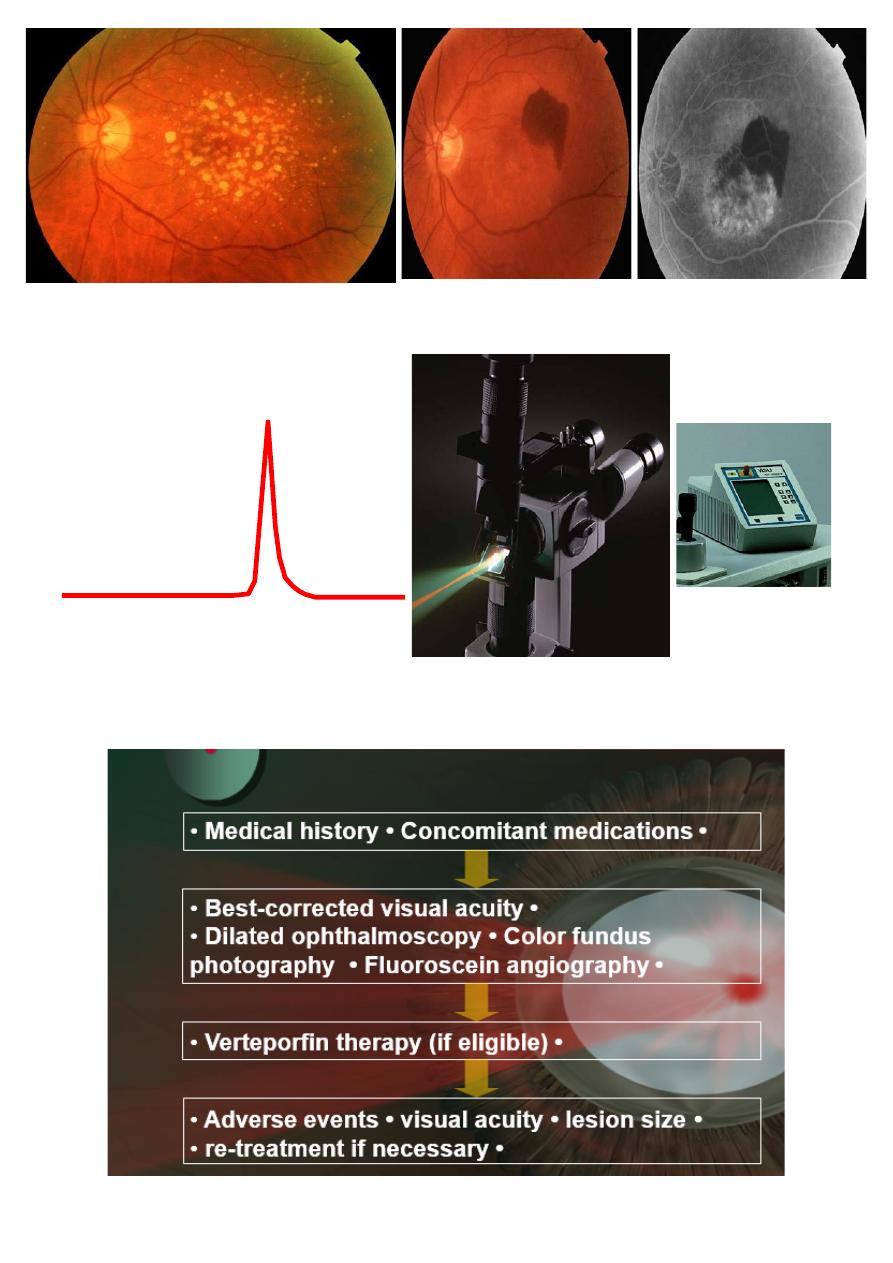
8
Principles of laser setup in PDT:
Overview of verteporfin therapy procedure:
9
Retinal detachment:
• Definition:
A separation of neruroretina from the underlying retinal pigment
epithelium (RPE) by the accumulation of subretinal fluid (SRF).
Rhegmatogenous retinal detachment:
Etiology: a retinal tear develops because of vitreoretinal traction on a
weak area in the peripheral retina (lattice degeneration).
Clinical features:
1-acute: mobile convex, slightly opaque, corrugated detached retina
with breaks.
2-long-lasting: retinal thinning, cysts, demarcation lines (high water
content), and fibrotic and immobile retina (proliferative
vitreoretinopathy).
Management:
1- Scleral buckling for uncomplicated cases.
2- Vitrectomy combined with intravitreal injection of gas or silicon
oil for complicated cases.
Tractional retinal detachment:
Contraction of fibrous tissue, e.g. associated with proliferative
diabetic retinopathy, causes the retina to detach without a break; on
examination, concave immobile retina with shallow SRF is seen.
Treated by pars plana vitrectomy (PPV).
Exudative retinal detachment:
Etiology: the passage of fluid from the choroid into the sub retinal
space occurs following breakdown of physiological barriers. Causes
include intraocular tumors, inflammation, sever CNV, extensive laser
photocoagulation and sever hypertension.
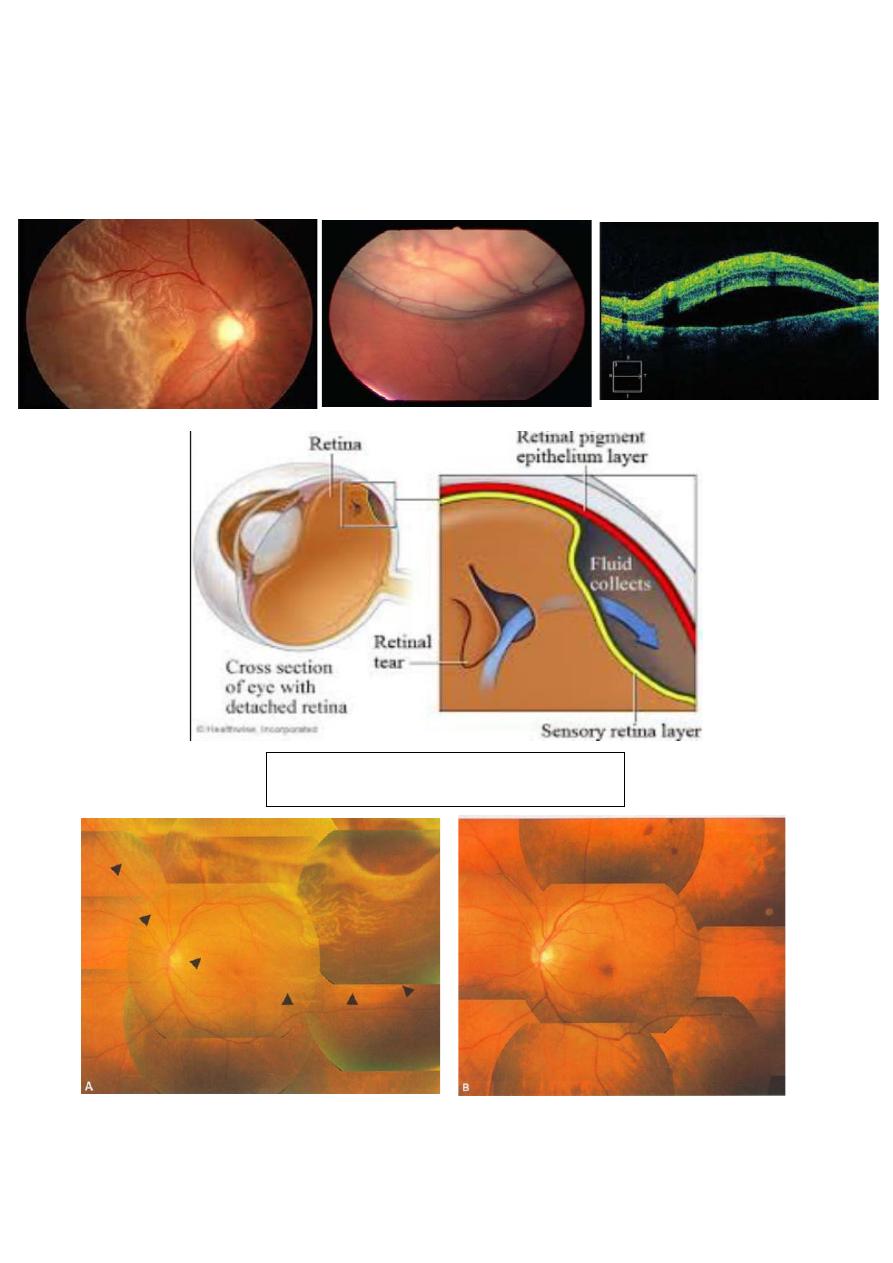
10
Clinical features: convex, very mobile retina with deep shifting fluid
and absence of retinal breaks.
Treatment consists of addressing the cause.
Retinal detachment repair
11
Diabetic retinopathy (DR):
Diabetic retinopathy (DR) is the most common cause of blindness in
the working-age population. The incidence and severity of DR are
strongly related to duration of diabetes; good control of blood glucose
and hypertension are very important.
Clinical features:
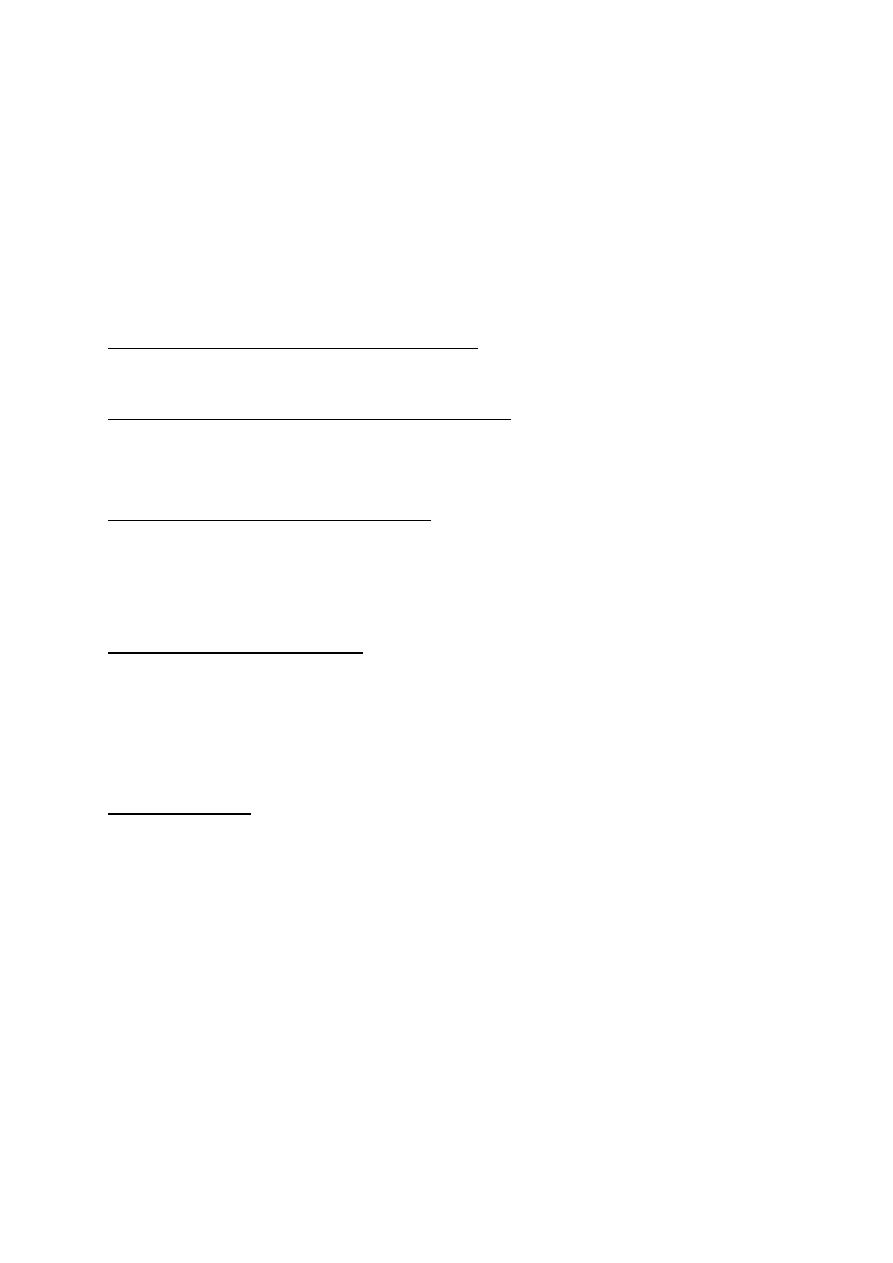
Background diabetic retinopathy (DR): micro aneurysms dot and blot
hemorrhages and hard exudates.
Pre-proliferative diab. Retinopathy (DR): Cotton wool spots,
intraretinal microvascular anomalies (IRMA), venous changes
(beading, looping and segmentation) and dark blot hemorrhages.
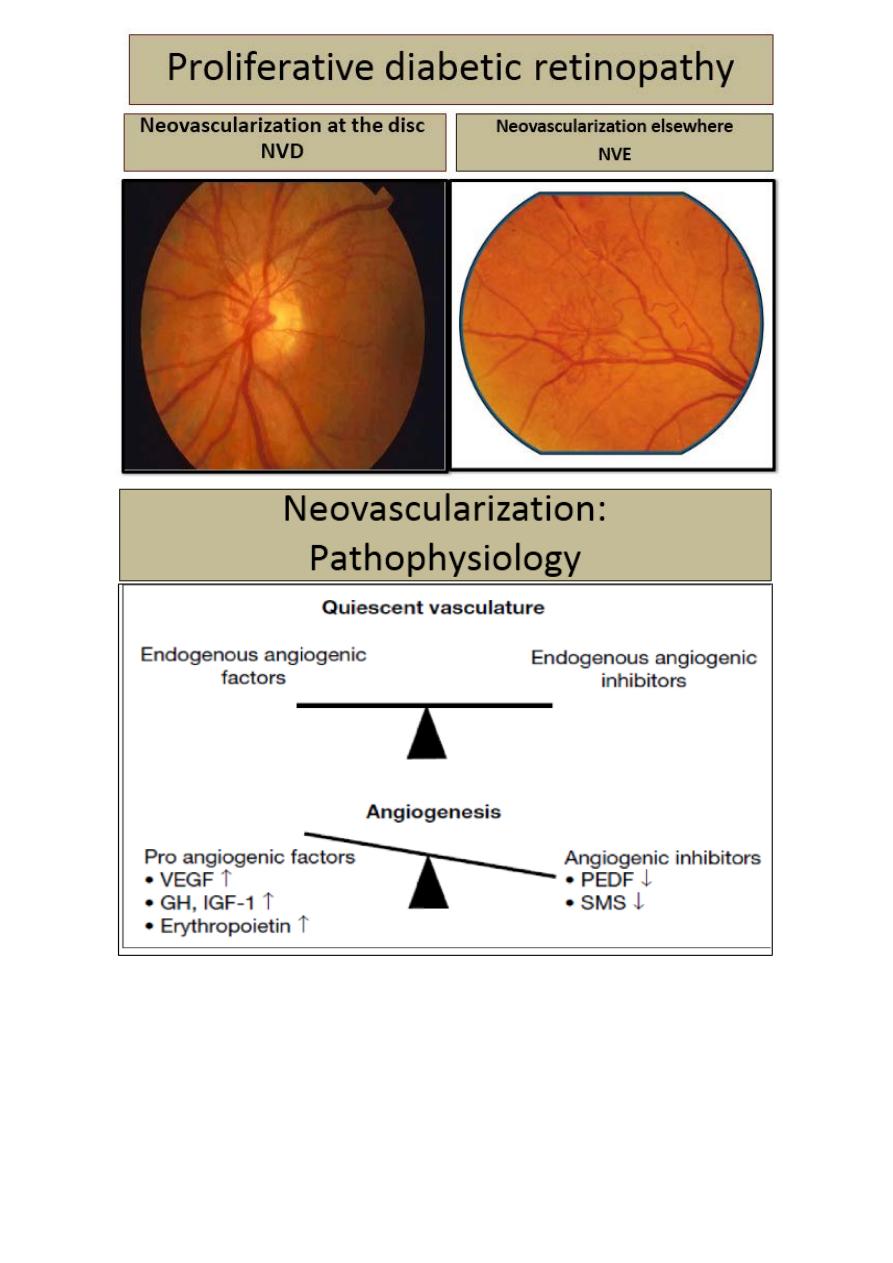
Proliferative diabetic retinopathy new vessel formation at the optic
disc (NVD) or elsewhere on the retina (NVE). Sever visual loss may
occur because of vitreous hemorrhage or tractional retinal detachment
due to contraction of fibro vascular tissue.
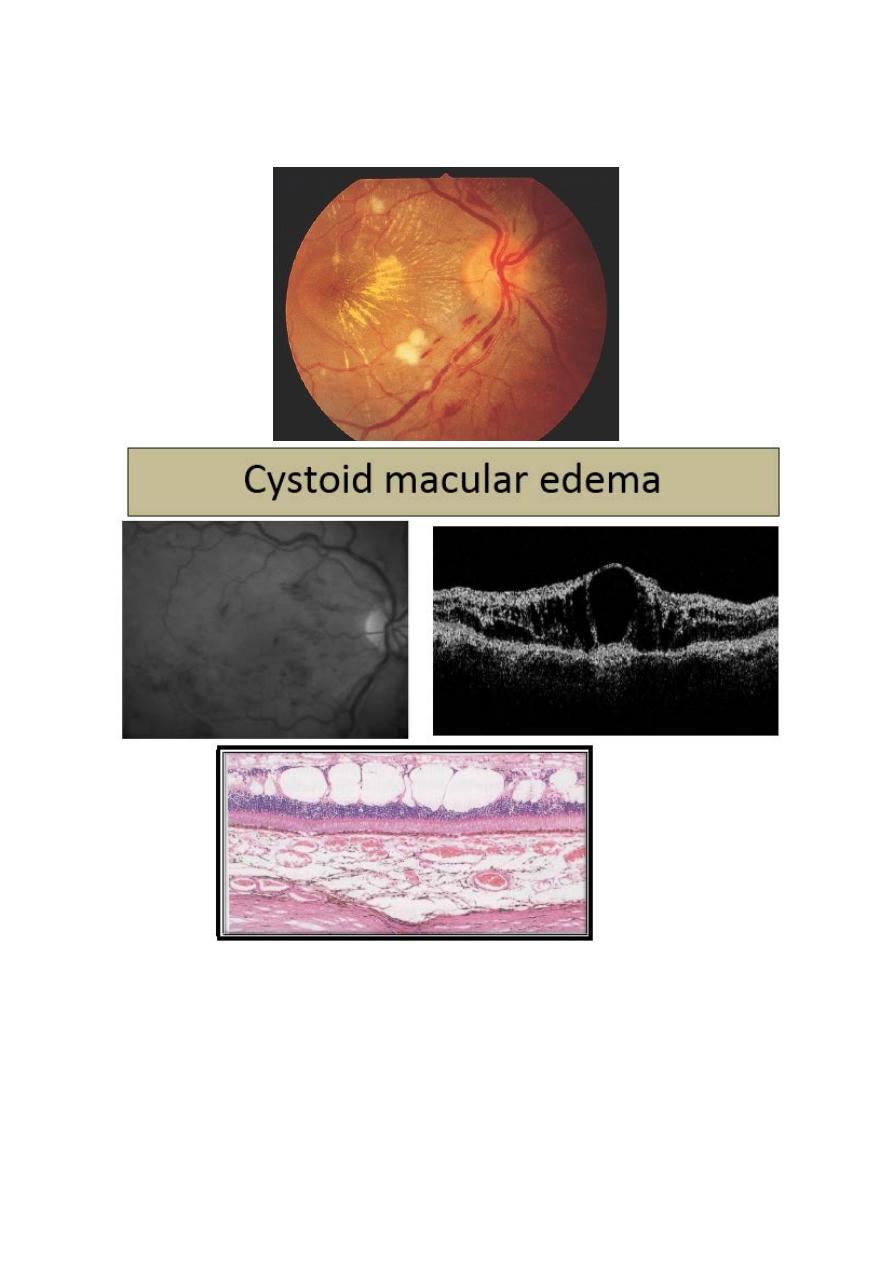
Diabetic maculopathy: is the most common cause of visual
impairment in patients with diabetes. Loss of visual function is
usually caused by edema, typically accompanied by exudates. Less
commonly, the macula becomes ischemic; often with sever
deterioration in central vision.
Management:
1- Regular review if treatment is not indicated frequency dependent
on severity of DR.
2- Pan retinal laser photocoagulation for proliferative DR
3- Grid or focal laser photocoagulation for macular edema fitting
certain criteria (clinically significant macular edema).
4- Vitrectomy for persistent vitreous hemorrhage or tractional retinal
detachment involving the center of macula.
5- Intravitreal Anti-vascular endothelial growth factor VEGFs in
selected cases e.g. Ranizumab (Avastin) and Bevazumab (Lucentis).
12
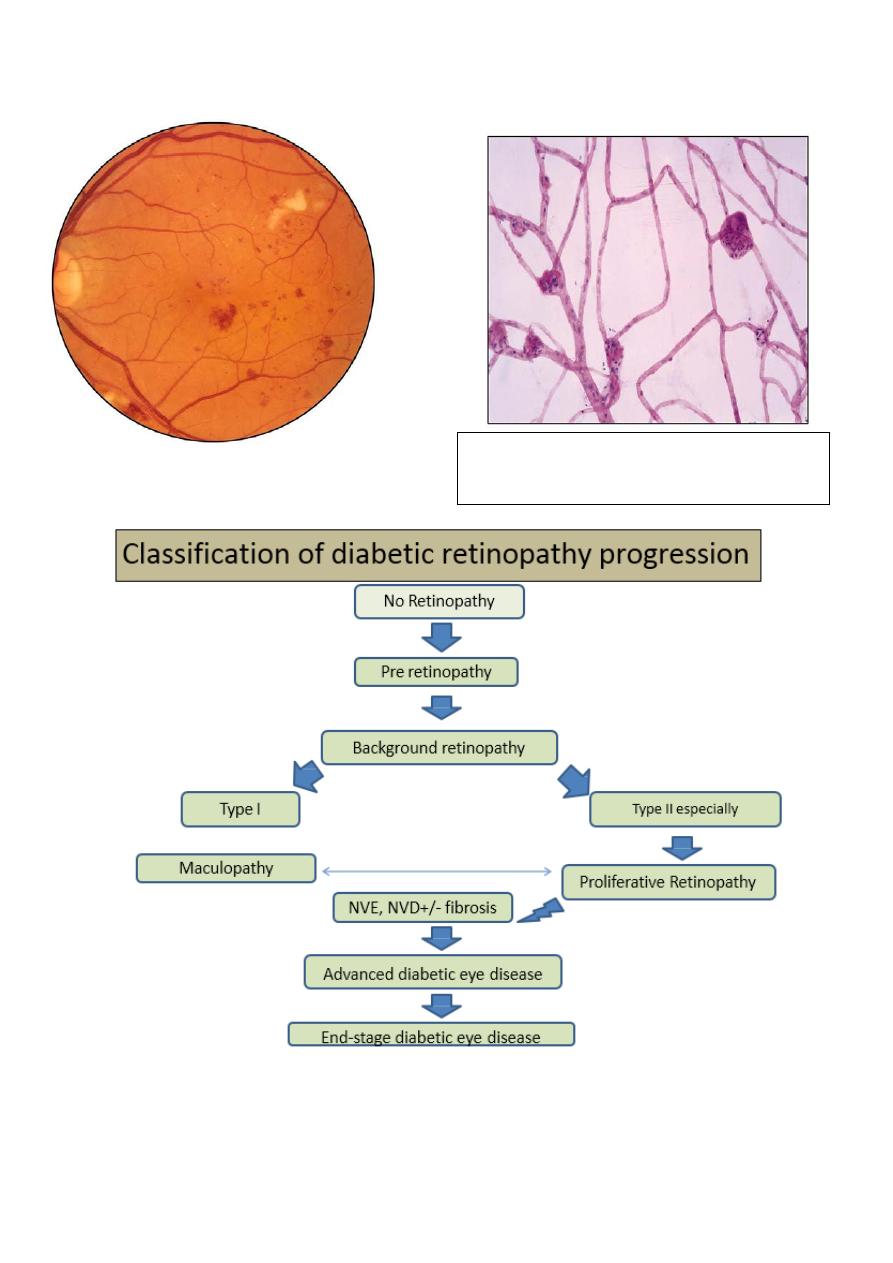
Degenerated pericytes which are
eosinophilic- trypsin digest preparation
13
14
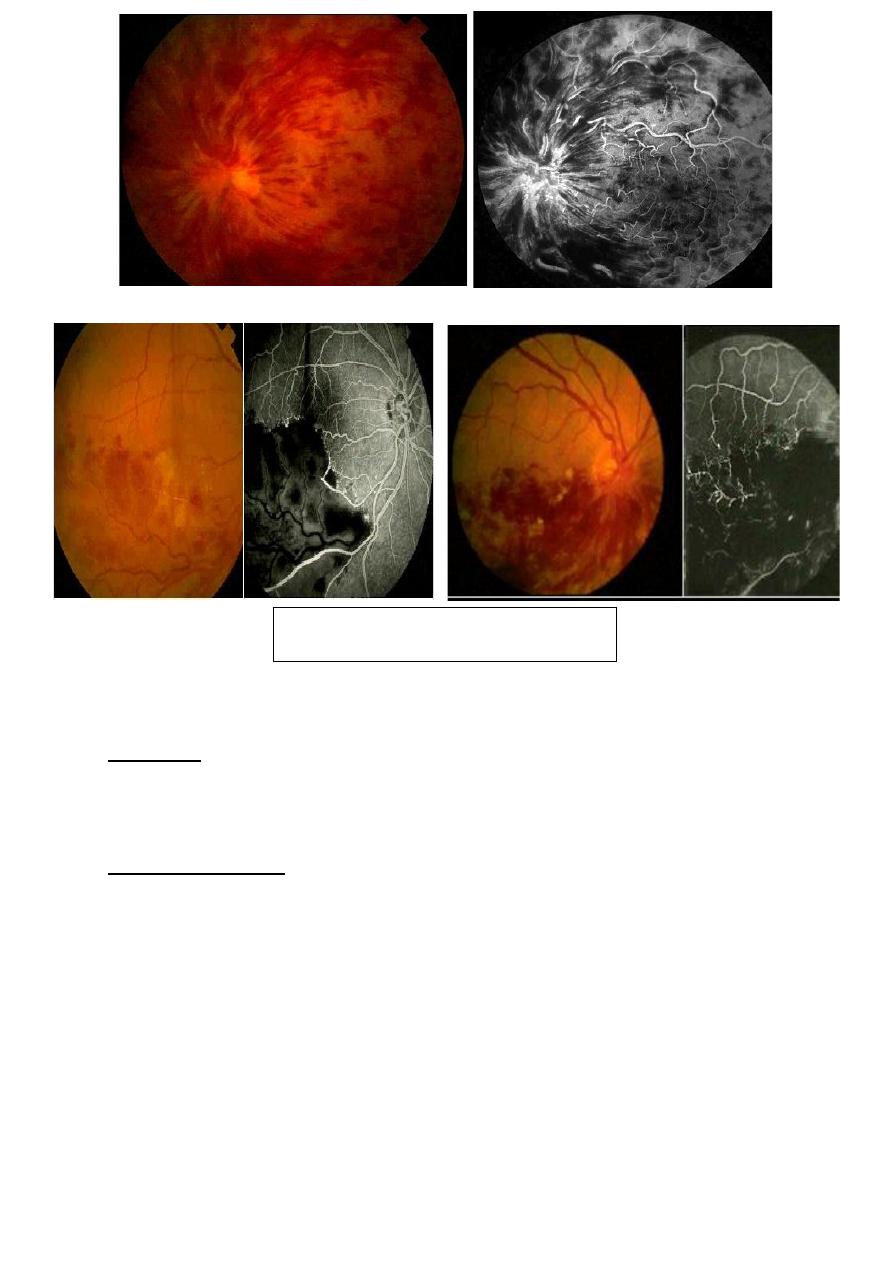
Retinal vein occlusion:
Retinal vein occlusion RVO:
Etiology:
Predisposing factors include;
1- Increasing age.
2- Hypertension.
3- Hyper viscosity.
4- Vasculitis.
5- Thrombophilic disorders.
6- Raised IOP.
Clinical features:
Presents with sudden mild to severe loss of vision in one eye. Acute
signs include hemorrhage, cotton wool spots, venous tortuosity, optic
disc and retinal edema.
Classification;
1- Branch retinal vein occlusion (BRVO): usually involve a retinal
quadrant.
2- Hemi- retinal vein occlusion.
3- Central RVO (CRVO).
Complications:
1- Retinal neovascularization, especially in BRVO, is treated with
laser photocoagulation.
2- Macular edema is treated with grid laser and/or anti-VEGF.
3- Neovascular glaucoma in ischemic CRVO treated with glaucoma
surgery with or without shunt device.
15
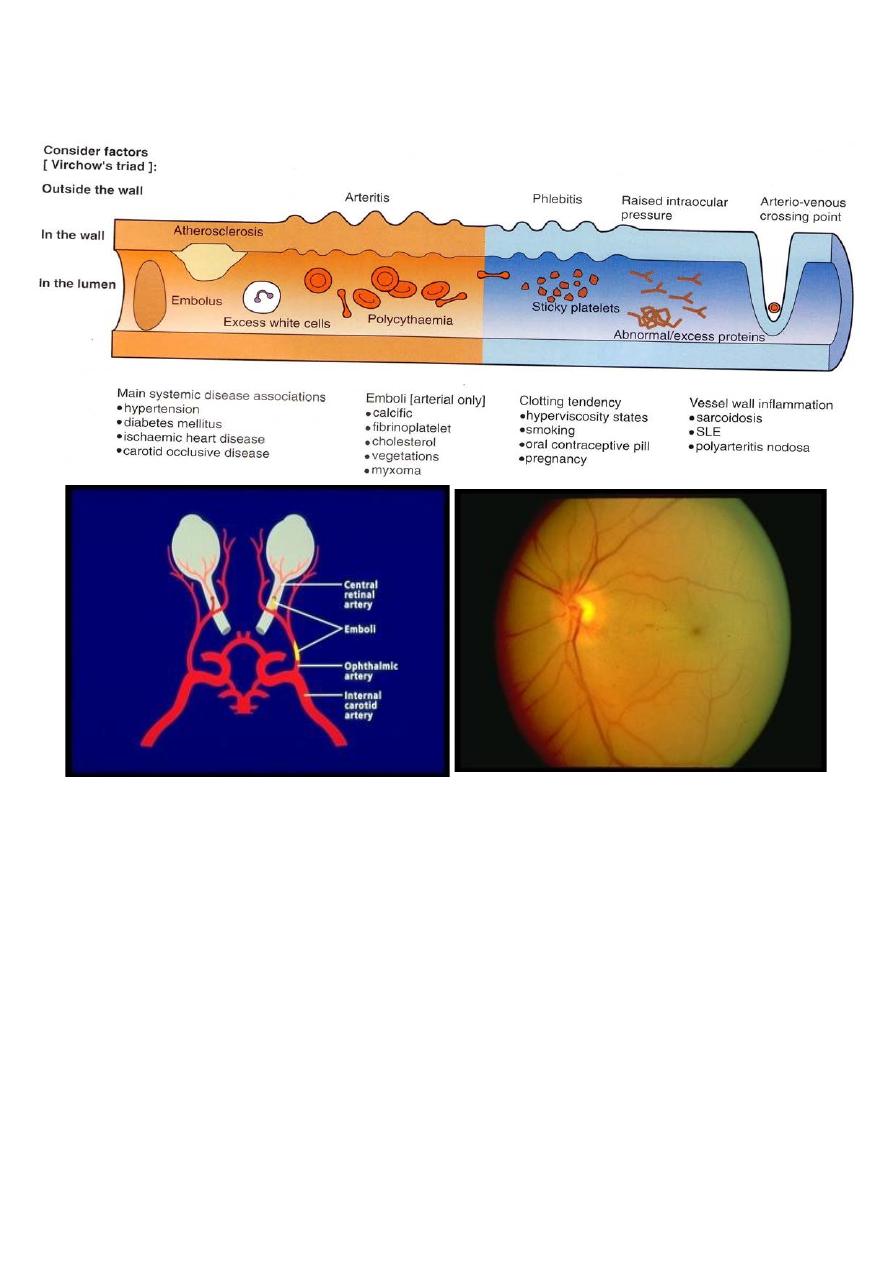
Retinal artery occlusion:
Etiology:
Embolization from a carotid or cardiac source, or vaso-obliteration by
atheroma or arteritis.
Clinical features:
Acute loss of vision; may be permanent or transient (amaurosis
fugax). Retinal pallor corresponding to the involved area (central or
branch) is seen, and in central RAO a “cherry red spot” at the fovea is
typically present. Segmentation of the arteriolar blood column (cattle
trucking) may be seen, Later the arterioles become attenuated and the
optic disc pale.
Branch retinal vein occlusion
16
Causes of retinal artery occlusion:
Central retinal artery occlusion treatment:
Re-breathing CO2.
Topical Beta blockers.
Intravenous acetozolamide 500 mg.
Massaging of globe with lids closed.
Anterior chamber paracentesis.
Calcium channel blockers.
Hyperbaric O2.
17
Amaurosis fugax:
ØMonocular dimming of vision.
ØTemporary arterial obstruction.
ØSudden, transient, painless visual
loss.
Amaurosis fugax evaluation:
Cardiovascular
Cerebrovascular
Ophthalmologic
Management:
1- Urgent ESR to exclude giant cell arteritis and investigation of
other risk factors
2- Amaurosis fugax: aspirin; carotid endarerectomy for sever
stenosis.
3- Acute RVO may be relieved by lowering IOP by massage,
intravenous acetazolamide, and anterior chamber paracentesis.
18
Hypertensive retinopathy
Toxoplasmosis:
Toxoplasma gondii is an obligate intracellular protozoan. The cat is
definitive host and other animals, such as mice and humans are
intermediate hosts.
1- Forms of parasite:
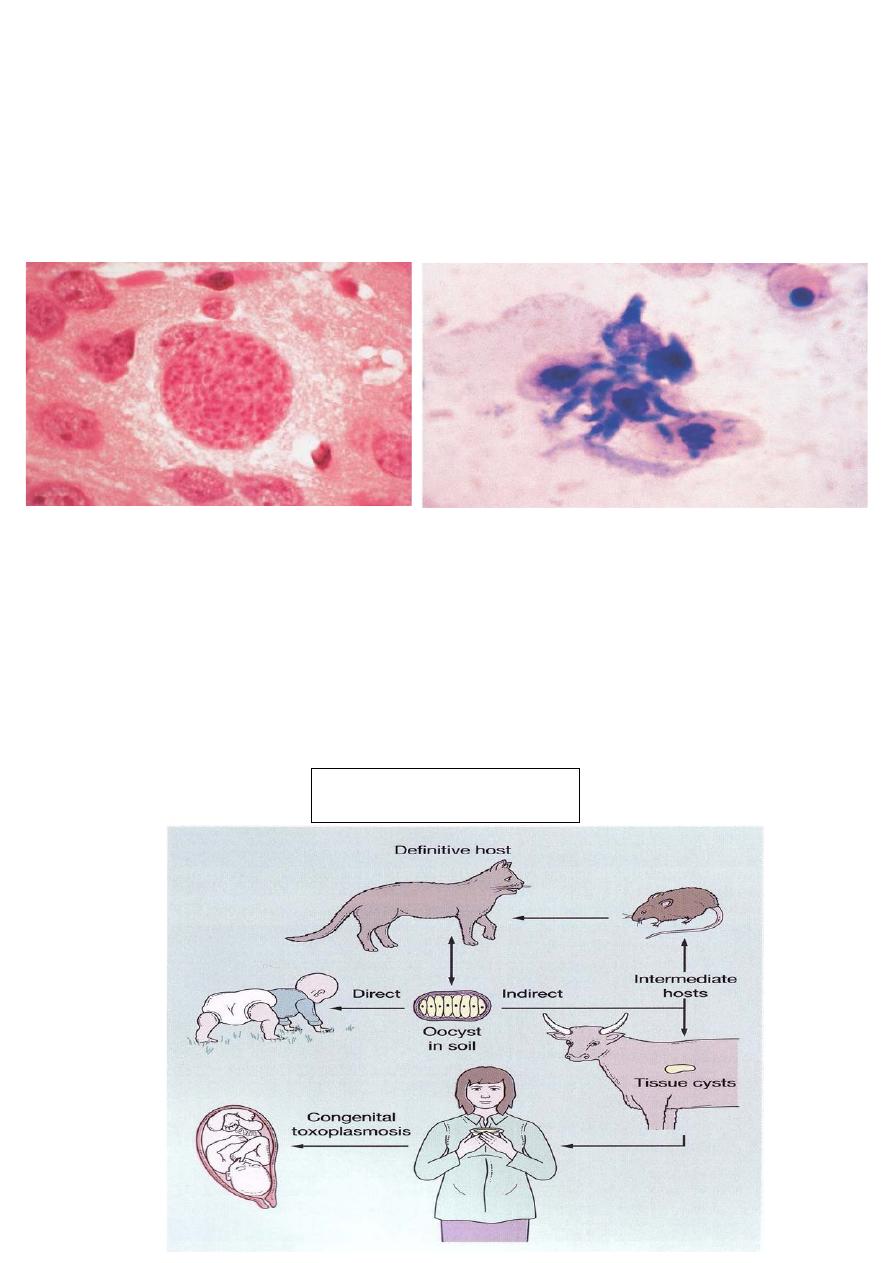
19
Sporocyst (o
ӧcyst) which is excreted in cat feces.
Bradzoite, which encysted in tissues.
Tachyzoite: is the active form, responsible for tissue destruction and
inflammation.
Ingestion of undercooked meat (lamb, beef) containing brabyzoites of
an intermediate host.
Ingestion of sporocyst following accidental contamination of hands
when disposing of cat litter trays and then subsequent transfer on to
food. Infants may become infested by eating dirt contains sporocyst.
Trans placental spread of the parasite (tachyzoite) can occur to the
fetus if a pregnant women become infested.
Ways of human infection
20
21
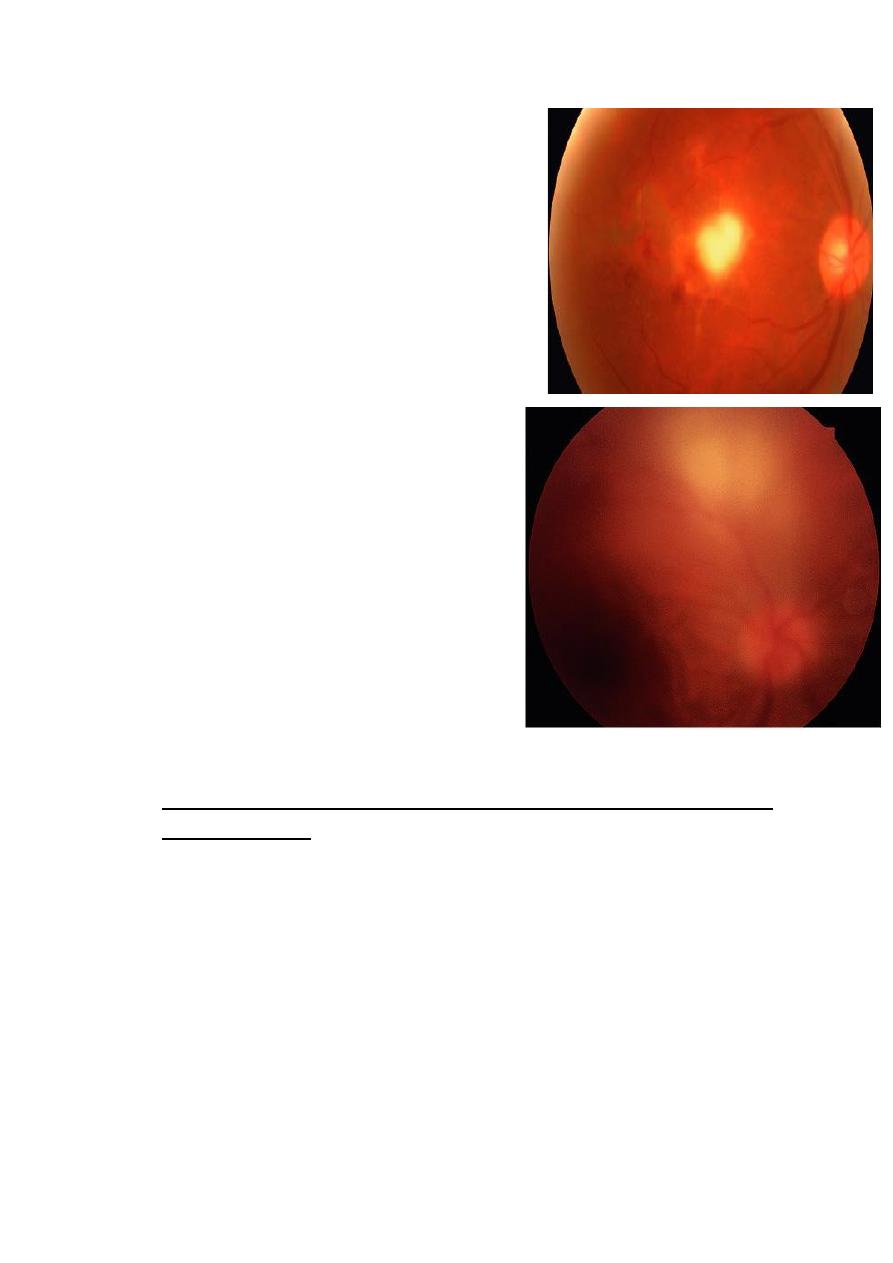
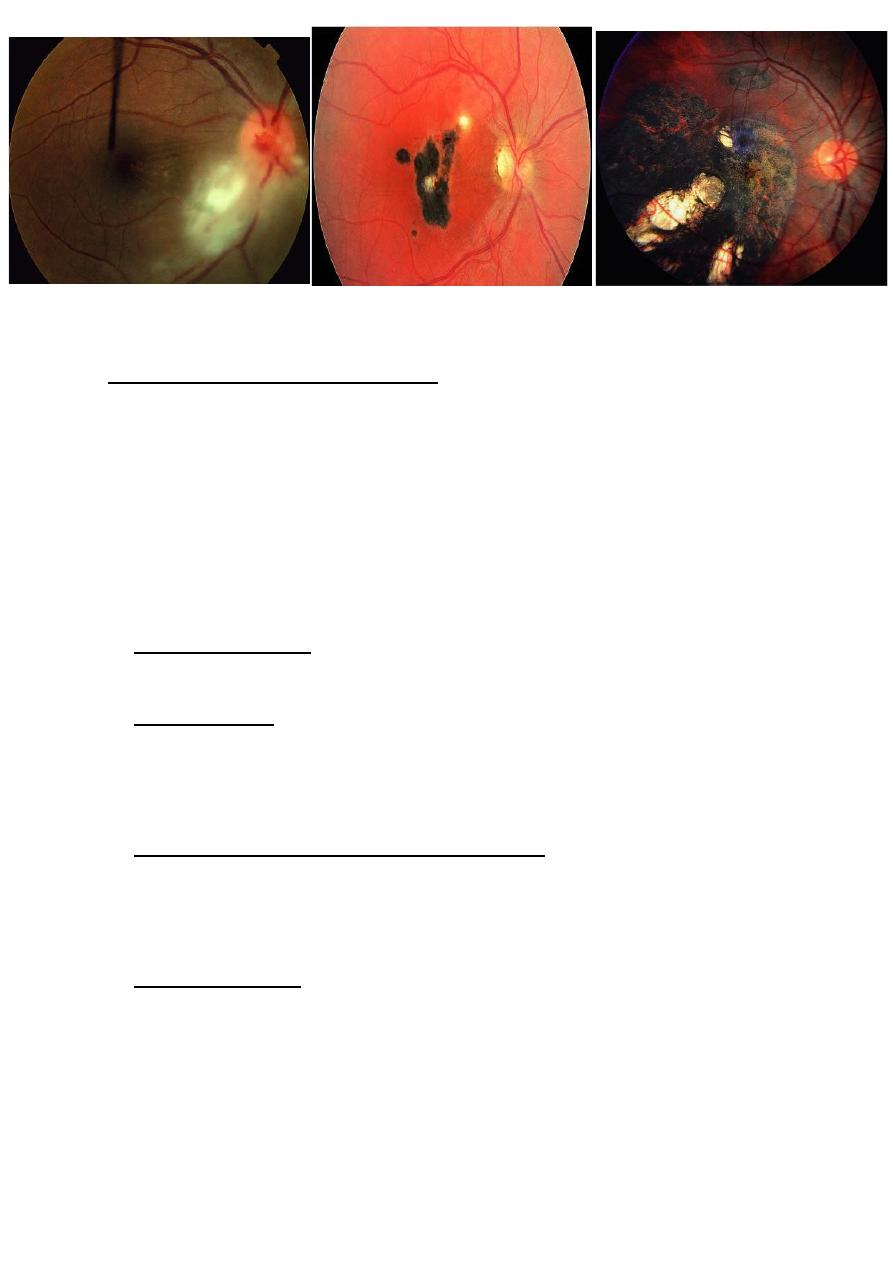
Clinical features of toxoplasma retinitis:
• Active retinitis is usually associated
with anterior uveitis which may be
non- granulomatous or
granulomatous.
• It is therefore very important to
examine the fundus in all patients
with anterior uveitis.
• Unilateral superficial retinitis: a
solitary inflammatory focus near an
old pigmented scar.
• The inflammatory focus may be
small or large and is associated
with an overlying vitreous haze.
• Very sever vitritis may greatly
impair visualization of fundus.
• Eyes with toxoplasmosis may lose vision from various direct or
indirect causes:
• Direct involvement : by an inflammatory focus of
fovea,papillomacular bundle, optic nerve head or a major blood
vessel.
• Indirect involvement by epiretinal or Vitreoretinal traction may
result in macular pucker or tractional retinal detachment.
22
Management of toxoplasma retinitis:
The main indication for treatment:
1 A lesion involving the macula, papillomacular bundle, optic
nerve head or major blood vessel.
2 A very sever vitritis because it may lead to vitreous fibrosis and
tractional retinal detachment.
3 In AIDS patients all lesions should be treated.
Therapeutic regimen:
1- Systemic steroids: are recommended in those with sever vitritis;
however, it is contraindicated in AIDS.
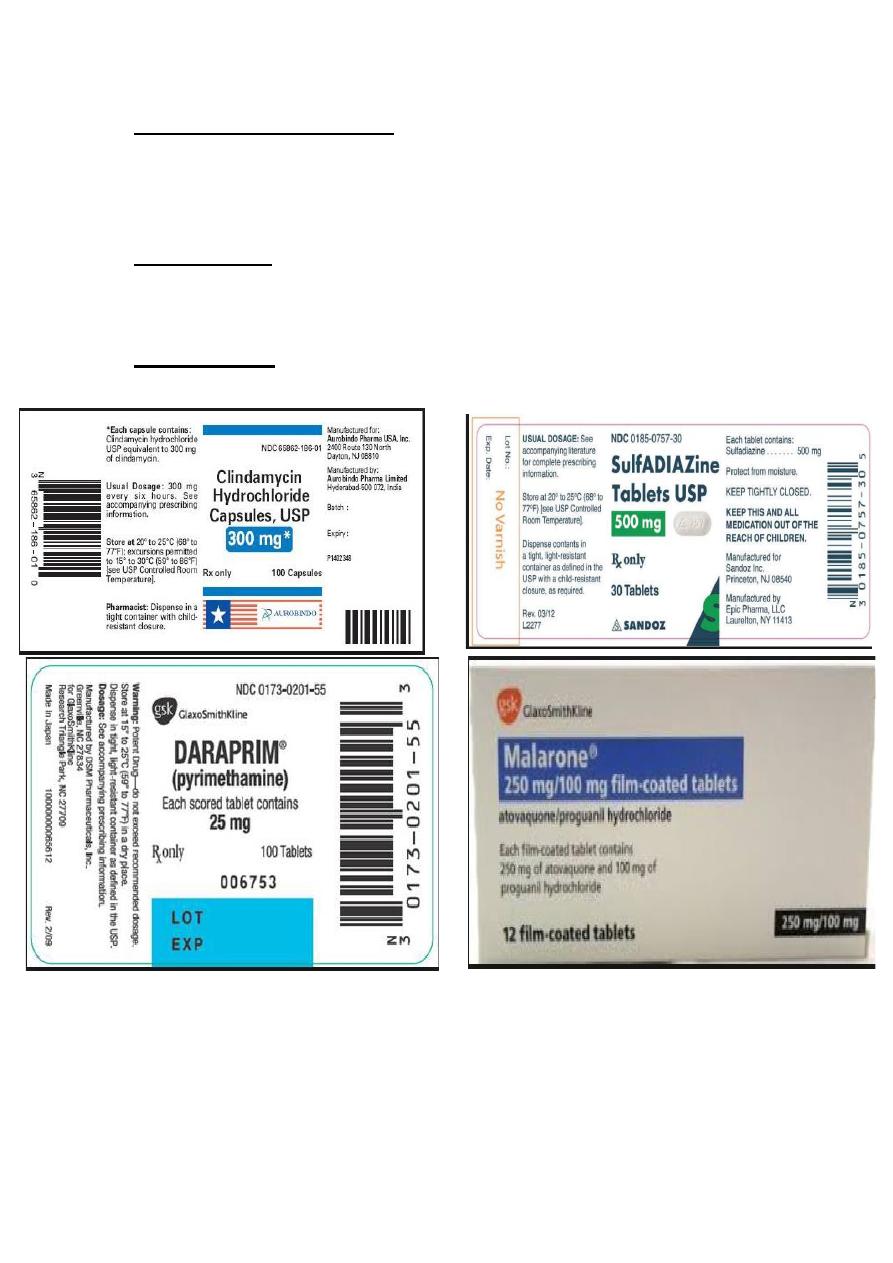
2- Clindamycin: 300 mg four times daily orally for three weeks, it
may cause pseudo membranous colitis secondary to clostridial
overgrowth, the risk of colitis is reduced when Clindamycin is used
together with a sulphonamide that inhibits clostridial overgrowth.
3- Sulphonamide therapy (sulphadiazine): the loading dose 2g
followed by 1g four times daily for 3-4 weeks, side effects of
sulphonamide include: renal stones, allergic reactions and Steven-
Johnson syndrome.
4- Pyrimethamine: ( is a strong anti-toxoplasma agent), which may
cause thrombocytopenia, leucopenia and folate deficiency, weekly
blood counts should be performed, and the drug used only in
combination with oral folonic acid. The loading dose is 50 mg
followed 25-50 mg daily for 4 weeks. Pyrimethamine should not be
used in patients with AIDS.
23
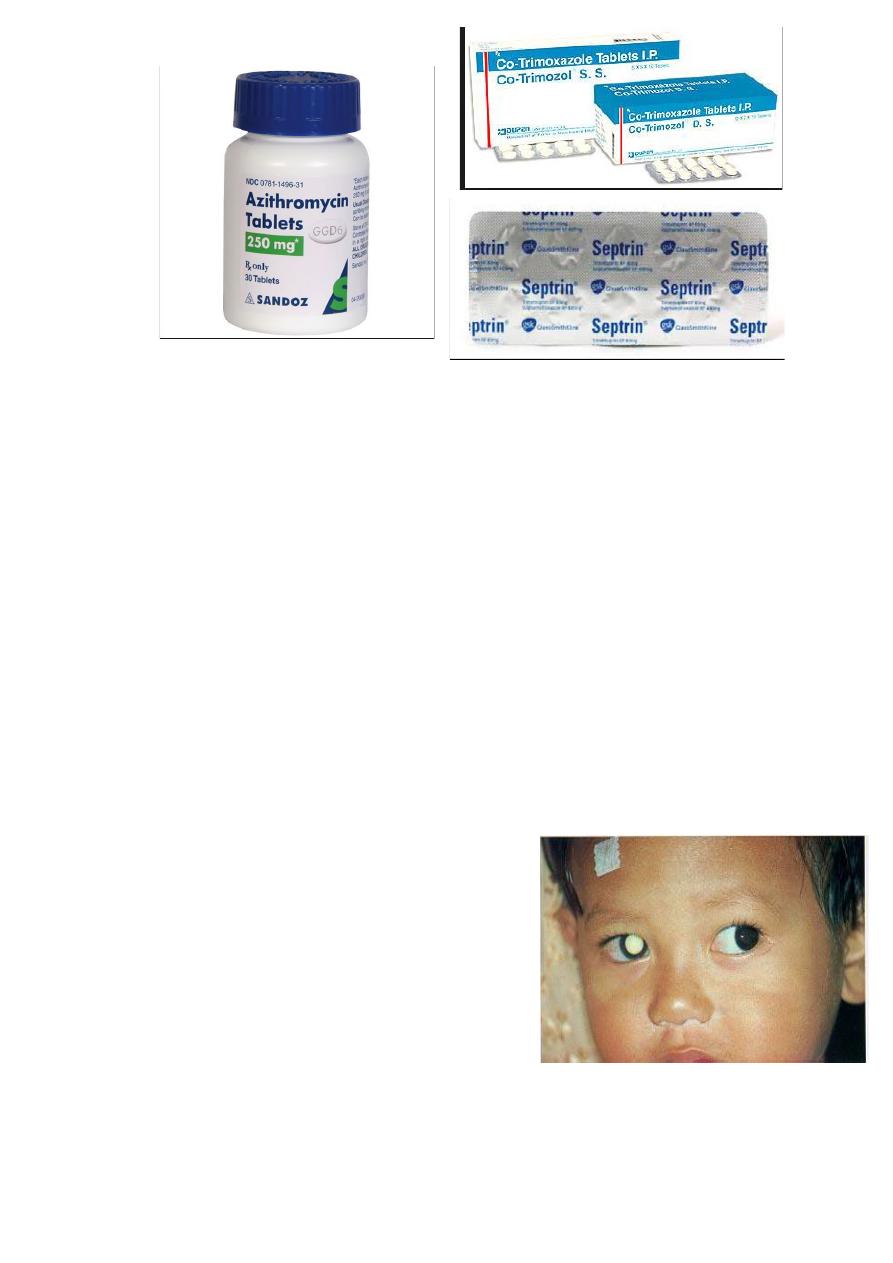
5- Co-trimoxazol (Septrin): consist of combination of trimethoprim
160 mg and sulphamethoxazole 800 mg, when used in oral doses of
960 mg twice-daily 4-6 weeks used alone or in combination with
clindamycin.
6- Atovaquone: 750 mg three times daily mainly used in treatment of
toxoplasmosis in AIDS. The drug is relatively free of serious side
effects, but it is expensive.
7- Azithromycin 500 mg daily on three successive days for those
who cannot tolerate other drugs.
24
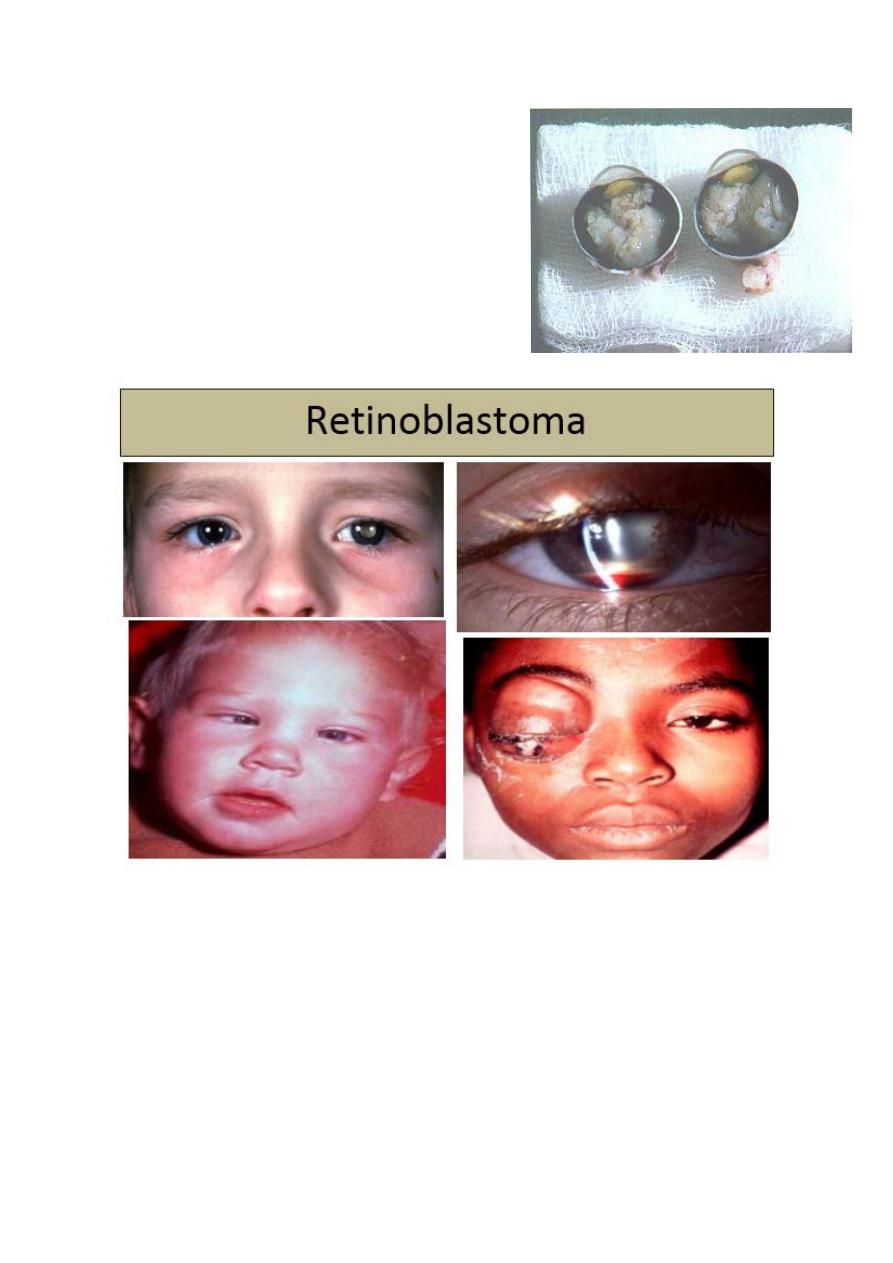
Retinoblastoma:
1. Important facts
2. Presentation
3. Signs
Endophytic
Exophytic
4. Treatment
5. Poor prognostic factors
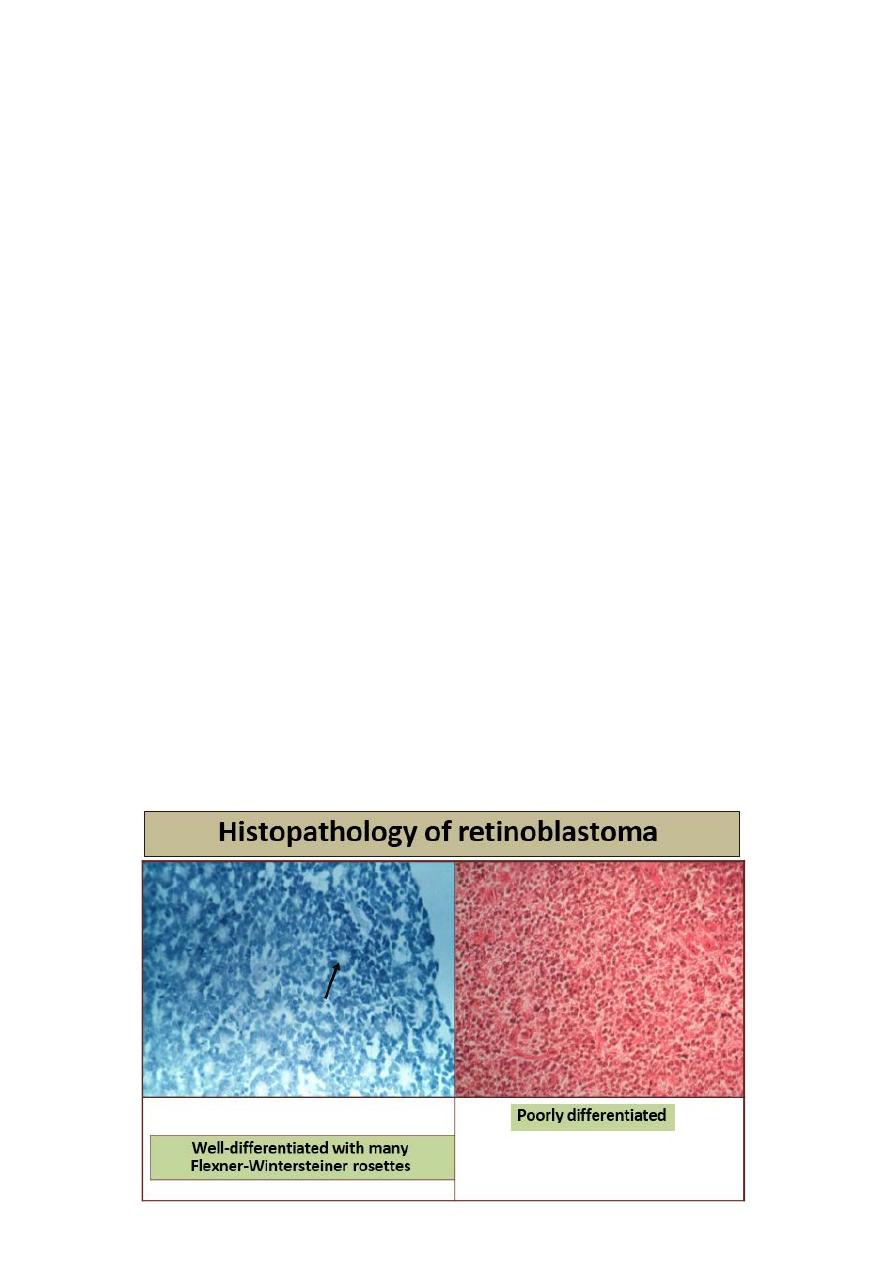
6. Histology
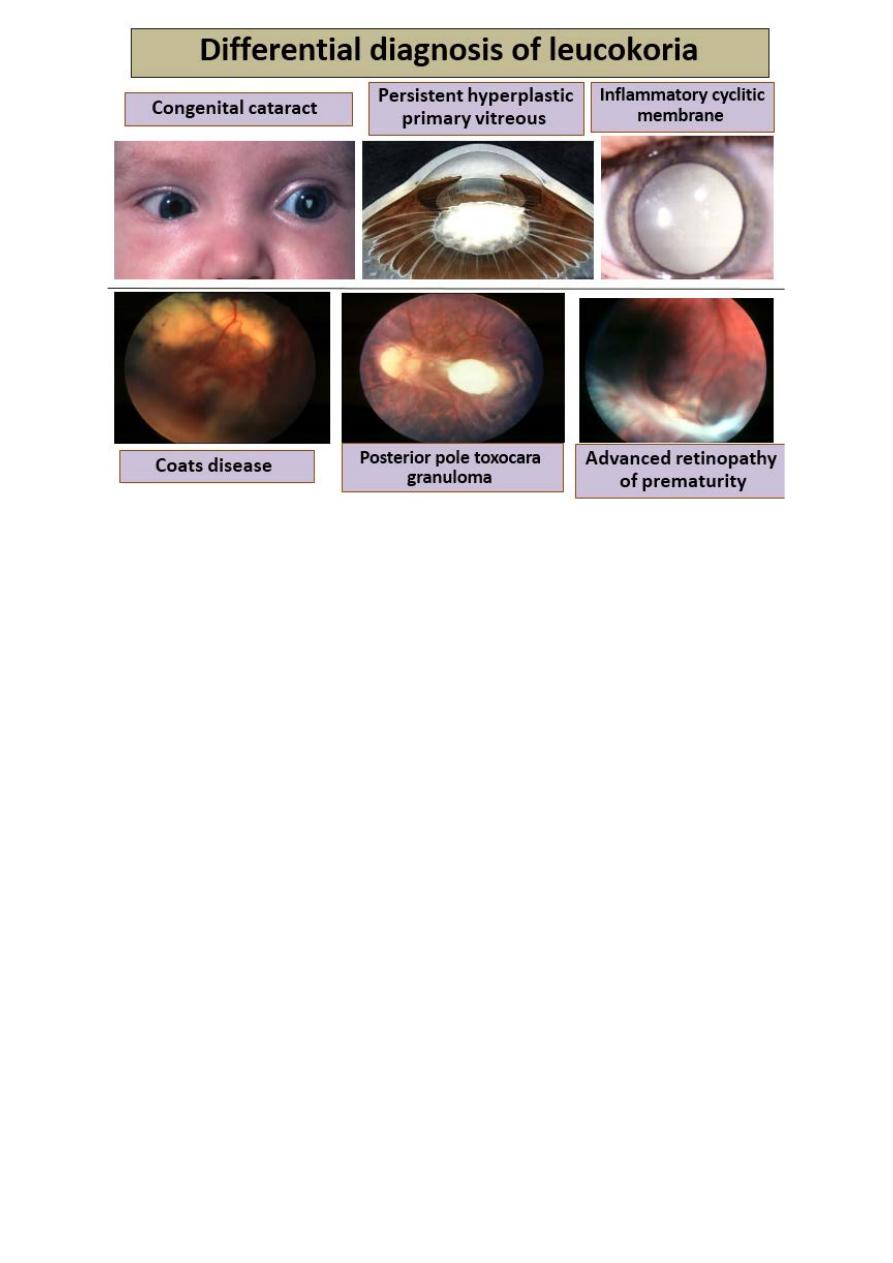
7. Differential diagnosis of leukocoria
Important facts:
1. Most common primary, malignant,
intraocular tumour of childhood
(1:20,000).
2. No sexual predilection.
3. Presents before age of 3 years
(average 3 months).
25
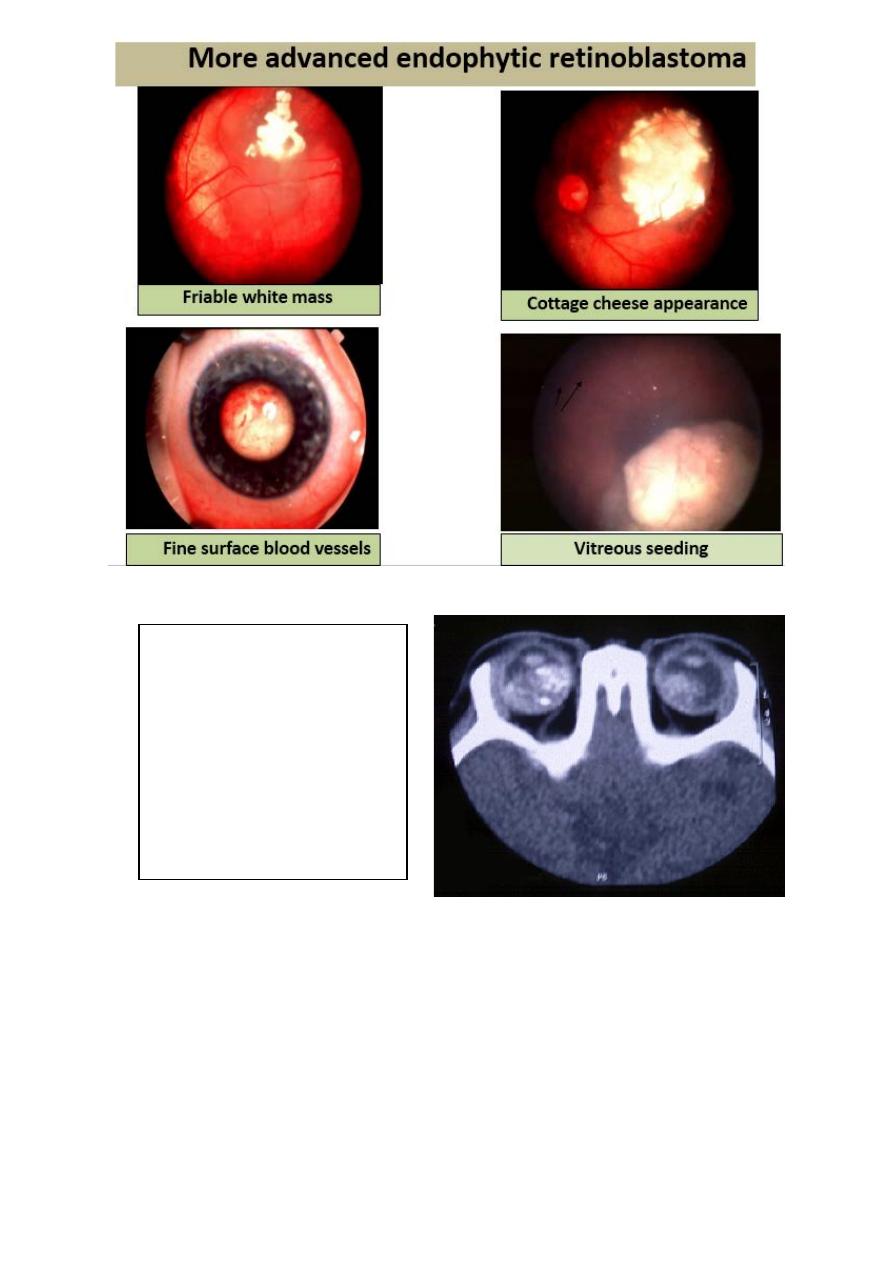
4. Heritable (40%) or non- heritable
(60%)
5. 5. Predisposing gene (RPE 1) on
13q14
26
CT diagnosis of retinoblastoma:
Treatment Options of Retinoblastoma:
1. Small tumors
Laser photocoagulation
Transpupillary thermotherapy
Cryotherapy
Medium tumors
calcification
• Optic nerve
involvement
• Orbital and CNS
extension
• Pinealoblastoma
27
Brachytherapy
Chemotherapy
External beam radiotherapy
Large tumors
Chemotherapy followed by local treatment
Enucleation
2. Extraocular extension
External beam radiotherapy
3. Metastatic disease
Chemotherapy
Poor Prognostic Factors in Retinoblastoma:
1. Optic nerve involvement
2. Choroidal invasion
3. Large tumour
4. Anterior location
5. Poor cellular differentiation
6. Older children
28
