Cell physiologyد.محمد عيىسى السبعاويدبلوم مفاصل وتأهيل طبيماجستير فسلجه طبية
Organization of the Cell
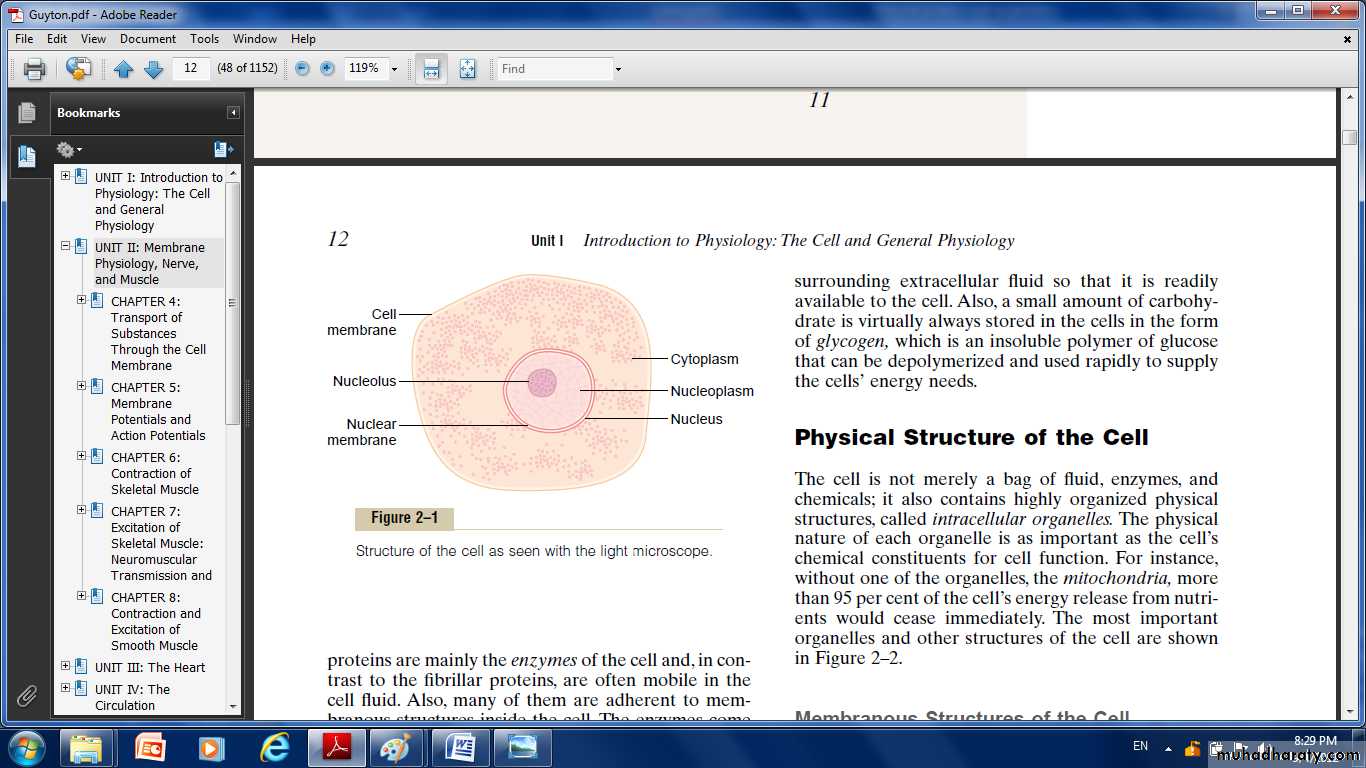
A typical cell formed by two major parts the nucleus and the cytoplasm.The nucleus is separated from the cytoplasm by a nuclear membrane.
The cytoplasm is separated from the surrounding fluids by a cell membrane, also called the plasma membrane.
The different substances that make up the cell are collectively called protoplasm.
Physical Structure of the Cell:
Membranous Structures of the CellThese membranes include the cell membrane, nuclear membrane, membrane of the endoplasmic reticulum, and membranes of the mitochondria, lysosomes, and Golgi apparatus.
Cell Membrane
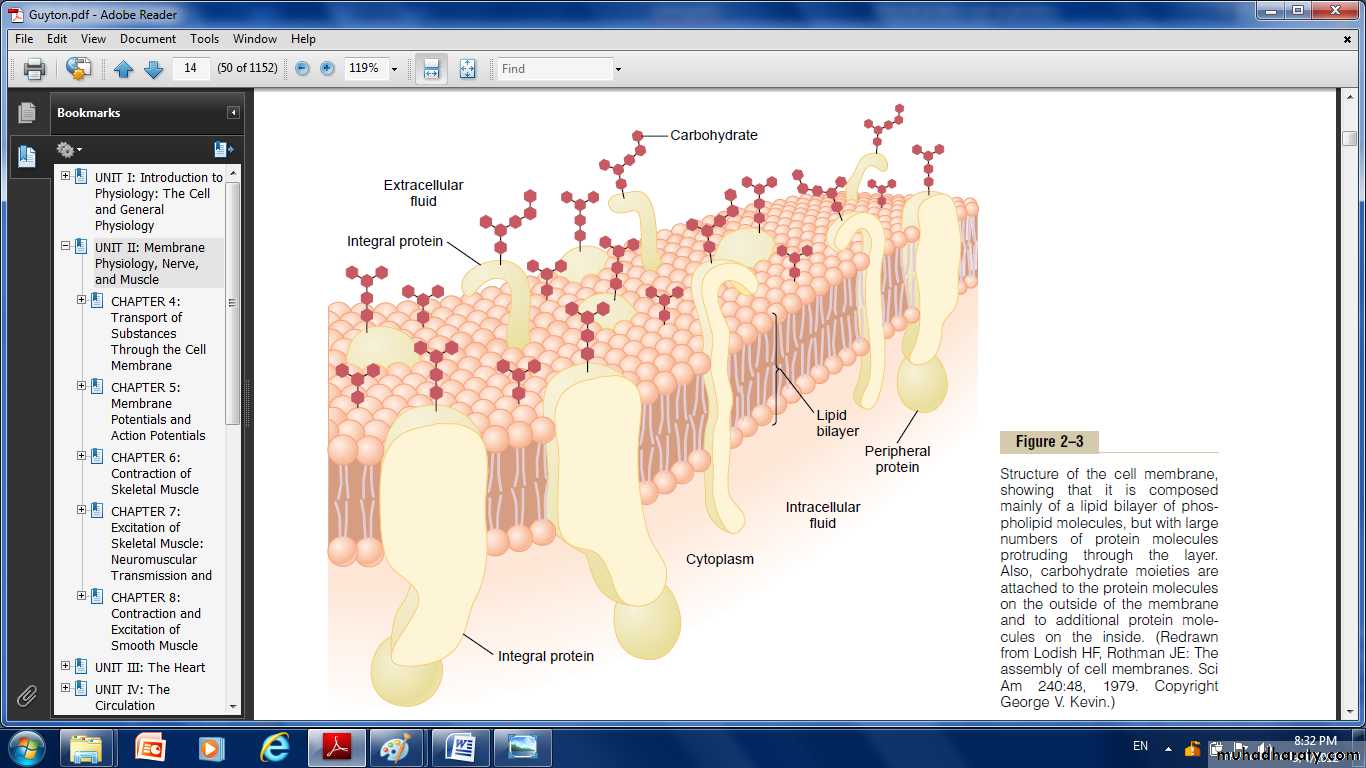
The cell membrane which envelops the cell, is a thin, pliable, elastic structure only 7.5 to 10 nanometers thick.The membrane composed almost entirely of proteins and lipids (proteins 55%; phospholipids 25%; cholesterol 13%; other lipids 4% and carbohydrates 3% ).
The lipids of the membranes provide a barrier that impedes the movement of water and water-soluble substances from one cell compartment to another because water is not soluble in lipids.
Protein molecules in the membrane often do penetrate all the way through the membrane, thus providing specialized pathways, often organized into actual pores, for passage of specific substances through the membrane.
Also, many other membrane proteins are enzymes that catalyze a multitude of different chemical reactions.
Lipid Barrier of the Cell Membrane Impedes Water Penetration:
The basic structure of cell membrane is a lipid bilayer, which is a thin, double-layered film of lipids.Interspersed in this lipid film are large globular protein molecules.
The basic lipid bilayer is composed of phospholipid molecules.
One end of each phospholipid molecule is soluble in water; that is hydrophilic.
The other end is soluble only in fats; that is hydrophobic.
The lipid layer of the membrane is impermeable to the usual water-soluble substances, such as ions, glucose, and urea.
Conversely, fat-soluble substances, such as oxygen, carbon dioxide, and alcohol, can penetrate this portion of the membrane with ease.
Cell Membrane Proteins.
There are globular masses floating in the lipid bilayer. These are membrane proteins, most of which are glycoproteins. Two types of proteins are present:integral proteins that protrude all the way through the membrane.
peripheral proteins that are attached only to one surface of the membrane and do not penetrate all the way through.
Many of the integral proteins provide structural channels (or pores) through which water molecules and water-soluble substances, especially ions, can diffuse between the extracellular and intracellular fluids.
These protein channels also have selective properties that allow preferential diffusion of some substances over others.
Other integral proteins act as carrier proteins for transporting substances that otherwise could not penetrate the lipid bilayer.
Sometimes these even transport substances in the direction opposite to their natural direction of diffusion, which is called “active transport.”
Still other integral protein act as enzymes.
Integral proteins can also serve as a receptors for water-soluble chemicals, such as hormones, that do not easily penetrate the cell membrane.
integral proteins spanning the cell membrane provide a means of conveying information about the environment to the cell interior.
Peripheral protein molecules are often attached to the integral proteins, their function almost entirely as enzymes or as controllers of transport of substances through the cell membrane“pores.”
Membrane Carbohydrates—The Cell “Glycocalyx.”
Membrane carbohydrates occur almost invariably in combination with proteins or lipids in the form of glycoprotein or glycolipids.The entire outside surface of the cell often has a loose carbohydrate coat called the glycocalyx.
The carbohydrate moieties attached to the outer surface of the cell have several important functions:
(1) Many of them have a negative electrical charge, which gives most cells an overall negative surface charge that repels other negative objects.
(2) The glycocalyx of some cells attaches to the glycocalyx of other cells, thus attaching cells to one another.
(3)Many of the carbohydrates act as receptor substances for binding hormones, such as insulin; when bound, this combination activates attached internal proteins that, in turn, activate a cascade of intracellular enzymes.
(4) Some carbohydrate moieties enter into immune reactions.
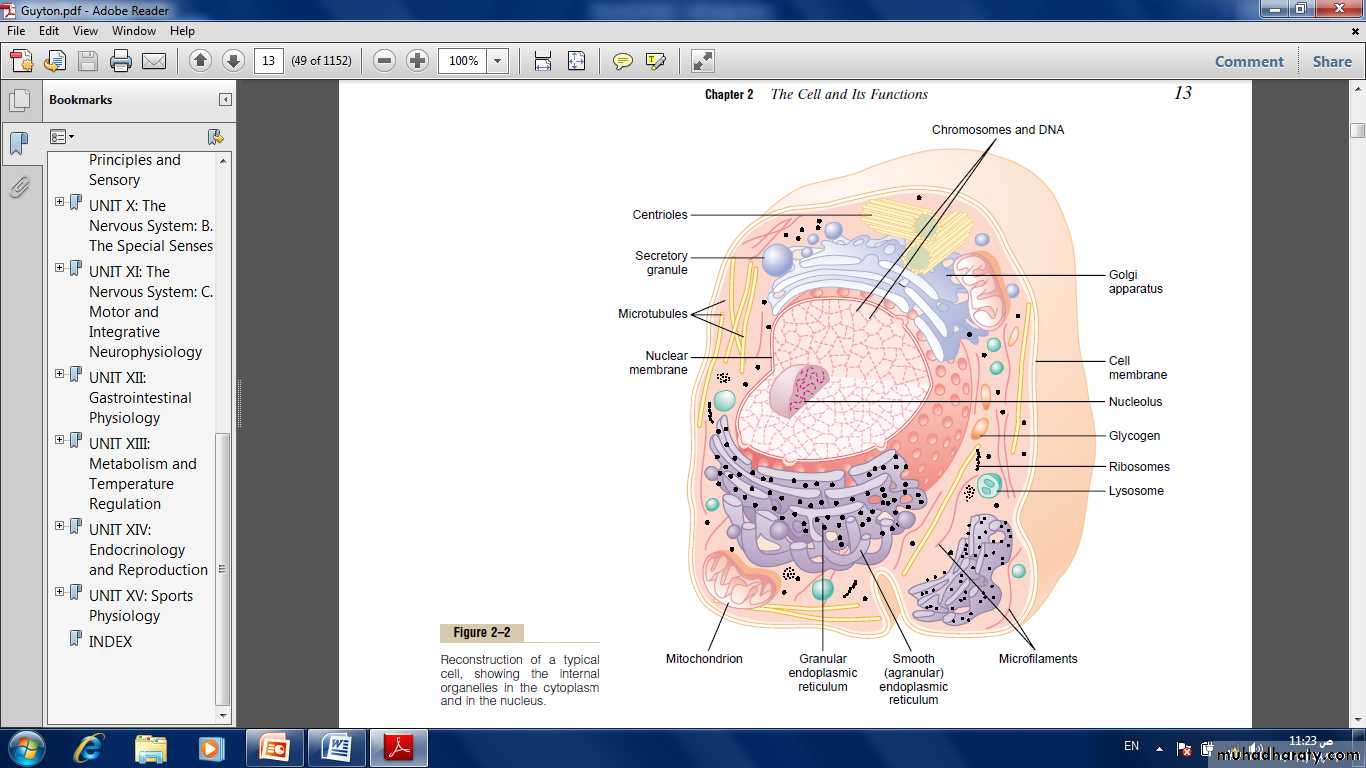
Cytoplasm and Its Organelles:
It consist of five important organelles:Endoplasmic reticulum.
The Golgi apparatus.
Mitochondria.
Lysosomes.
Peroxisomes.
Endoplasmic Reticulum
It's a network of tubular and flat vesicular structures in the cytoplasm; The tubules and vesicles interconnect with one another. Also, their walls are constructed of lipid bilayer membranes that contain large amounts of proteins.The space inside the tubules and vesicles is filled with endoplasmic matrix, a watery medium.
The space inside the endoplasmic reticulum is connected with the space between the two membrane surfaces of the nuclear membrane.
The vast surface area of this reticulum and the multiple enzyme systems attached to its membranes provide machinery for a major share of the metabolic functions of the cell.
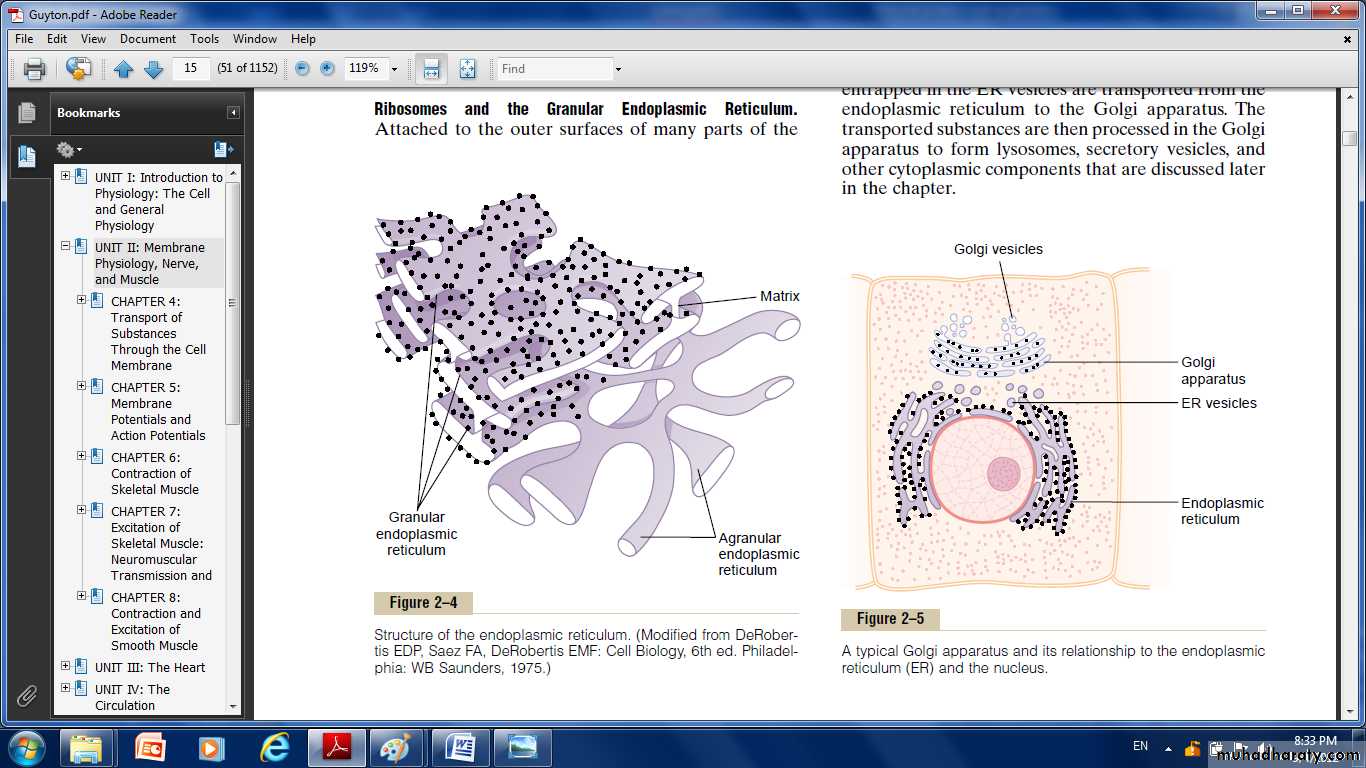
Ribosomes and the Granular Endoplasmic Reticulum.
Attached to the outer surfaces of many parts of the endoplasmic reticulum are large numbers of minute granular particles called ribosomes.Where these are present, the reticulum is called the granular endoplasmic Reticulum, they function to synthesize new protein molecules.
A granular Endoplasmic Reticulum.
Part of the endoplasmic reticulum has no attached ribosomes . This part is called the agranular, or smooth, endoplasmic reticulum.its functions is synthesis of lipid substances.
Golgi Apparatus:
Is closely related to the endoplasmic reticulum.It has membranes similar to those of the agranular endoplasmic reticulum.
It usually is composed of four or more stacked layers of thin, flat, enclosed vesicles lying near one side of the nucleus.
This apparatus is prominent in secretory cells, where it is located on the side of the cell from which the secretory substances are extruded.
The Golgi apparatus functions in association with the endoplasmic reticulum, small “transport vesicles” (endoplasmic reticulum vesicles) continually pinch off from the endoplasmic reticulum then fused with the Golgi apparatus. So, substances entrapped in the ER vesicles are transported to the Golgi apparatus.
The transported substances are then processed in the Golgi apparatus to form lysosomes, secretory vesicles and other cytoplasmic components
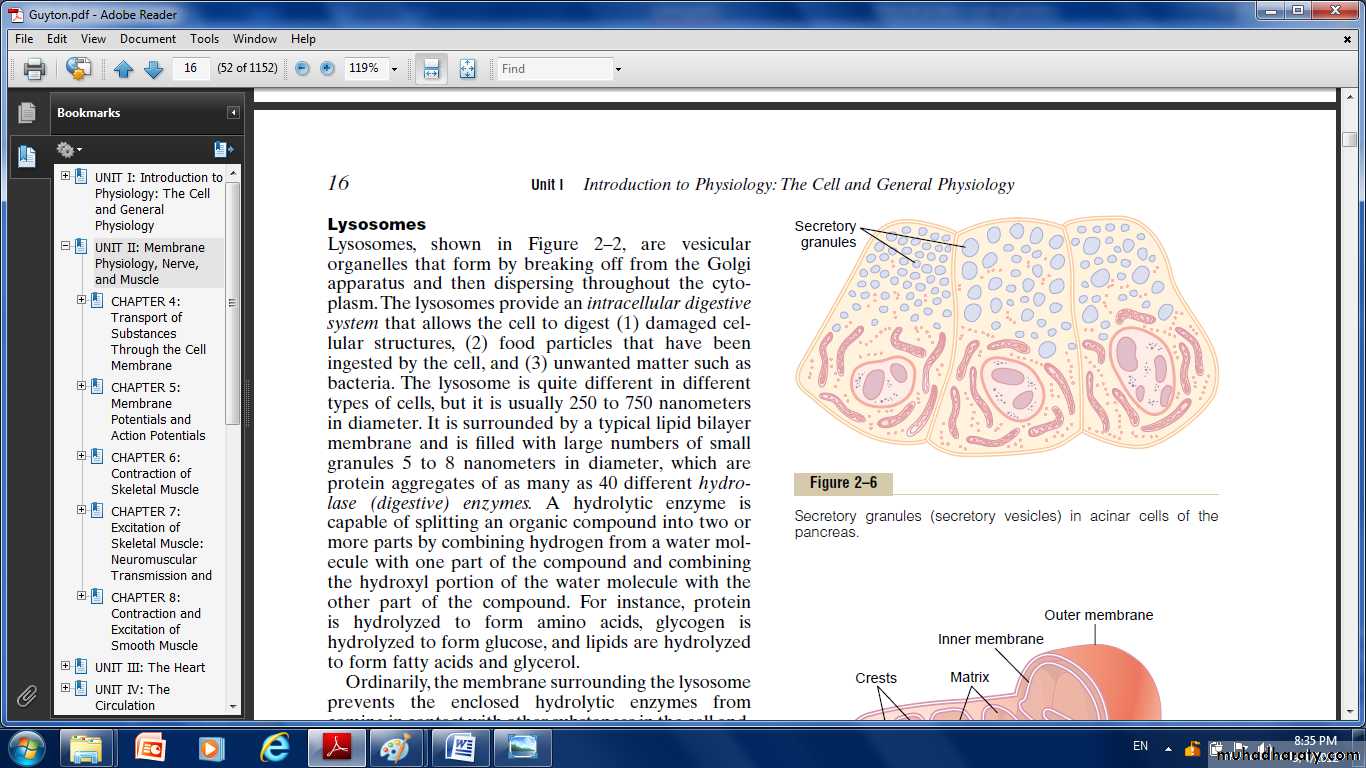
Lysosomes
Its vesicular organelles that form by breaking off from the Golgi apparatus and then dispersing throughout the cytoplasm.
The lysosomes provide an intracellular digestive system that allows the cell to digest:
(1) Damaged cellular structures.
(2) Food particles that have been ingested by the cell.
(3) Unwanted matter such as bacteria.
It is surrounded by a typical lipid bilayer membrane and is filled with large numbers of small granules, which are protein aggregates of as many as 40 different hydrolase (digestive) enzymes.
A hydrolytic enzyme is capable of splitting an organic compound into two or more parts(protein, glycogen, and lipids).
The membrane surrounding the lysosome prevents the hydrolytic enzymes from their digestive actions.
Peroxisomes
Peroxisomes are similar physically to lysosomes, but they are different in two important ways:First, they are believed to be formed by self-replication (or perhaps by budding off from the smooth endoplasmic reticulum) rather than from the Golgi apparatus.
Second, they contain oxidases rather than hydrolases. Several of the oxidases are capable of combining oxygen with hydrogen ions derived from different intracellular chemicals to form hydrogen peroxide (H2O2).
Hydrogen peroxide is a highly oxidizing substance and is used in association with catalase, another oxidase enzyme present in large quantities in peroxisomes, to oxidize many substances that might otherwise be poisonous to the cell.
Secretory Vesicles
One of the important functions of many cells is secretion of special chemical substances. Almost all such secretory substances are formed by the endoplasmic reticulum–Golgi apparatus system and are then released from the Golgi apparatus into the cytoplasm in the form of storage vesicles called secretory vesicles or secretory granules.Mitochondria:
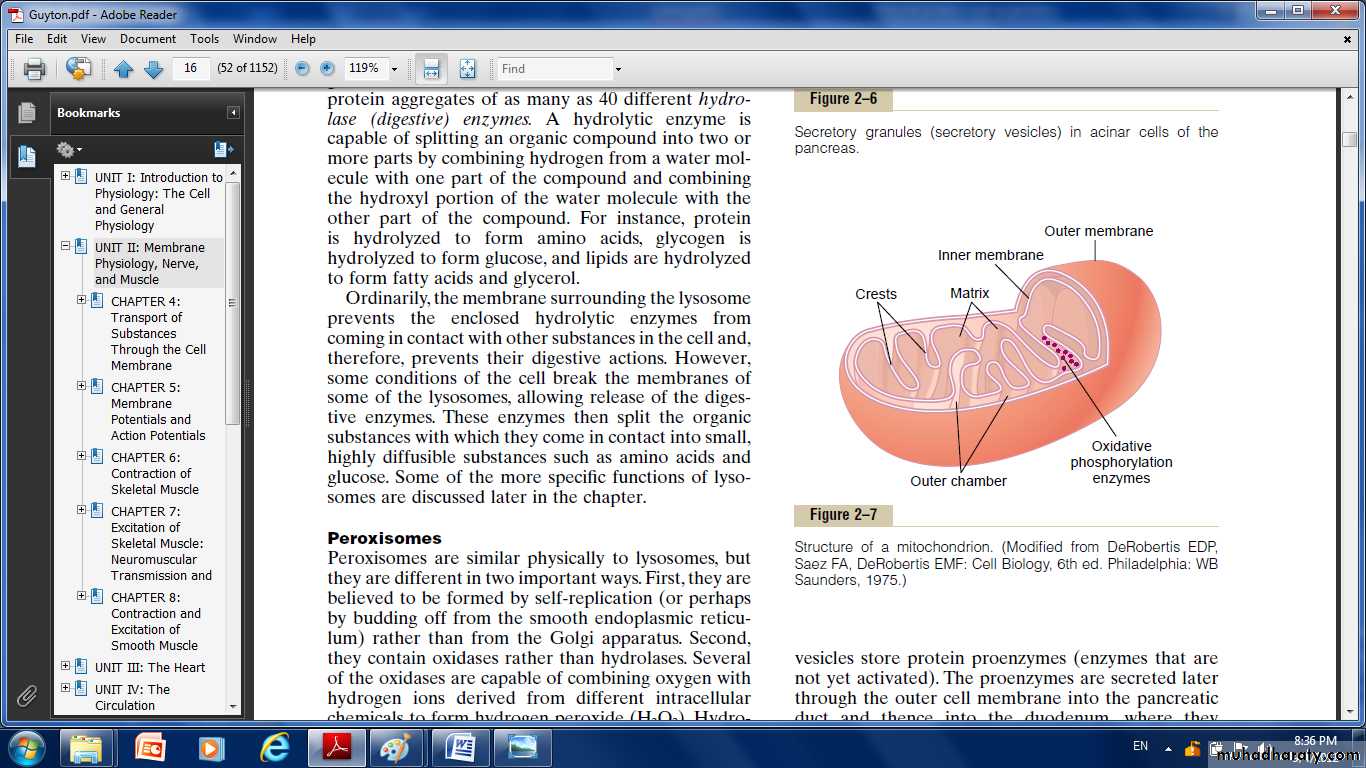
it also called the “power houses” of the cell.Without them, cells would be unable to extract enough energy from the nutrients.the mitochondria are concentrated in those portions of the cell that are responsible for the major share of its energy metabolism.
There number per cell varies from less than a hundred up to several thousand, depending on the amount of energy required by the cell.
They are also variable in size and shape. Some are only a few hundred nanometers in diameter and globular in shape, whereas others are elongated—as large as 1 micrometer in diameter and 7 micrometers long; still others are branching and filamentous.
It composed mainly of two lipid bilayer–protein membranes: an outer membrane and an inner membrane. Many infoldings of the inner membrane form shelves onto which oxidative enzymes are attached.
The inner cavity of the mitochondrion is filled with a matrix that contains large quantities of dissolved enzymes that are necessary for extracting energy from nutrients.
These enzymes operate in association with the oxidative enzymes on the shelves to cause oxidation of the nutrients, thereby forming carbon dioxide and water and at the same time releasing energy.
The liberated energy is used to synthesize a “high-energy” substance called adenosine triphosphate (ATP).
ATP is then transported out of the mitochondrion, and it diffuses throughout the cell to release its own energy wherever it is needed for performing cellular function.
Mitochondria are self-replicative, which means that one mitochondrion can form a second one, a third one,and so on, whenever there is a need in the cell for increased amounts of ATP.
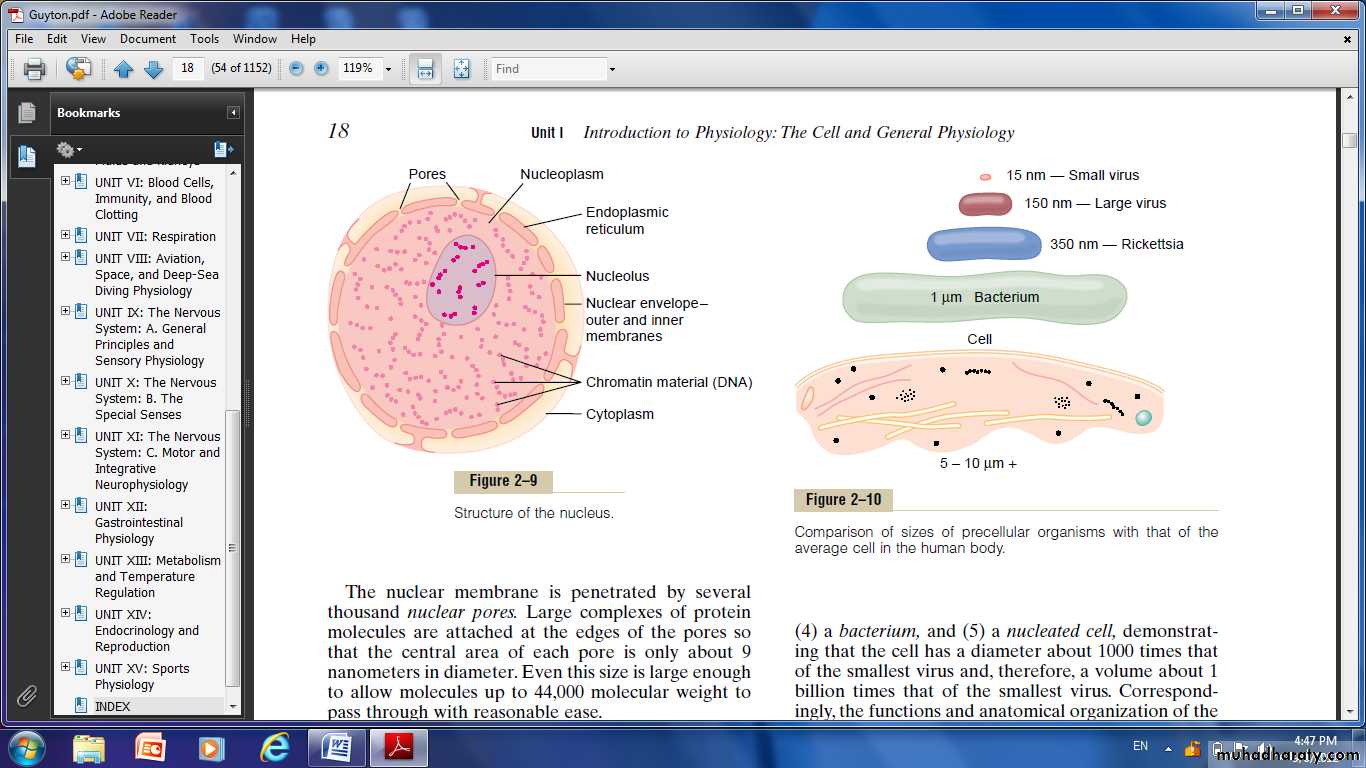
Nucleus:
The nucleus is the control center of the cell. It contains large quantities of DNA, which are the genes.The genes determine the characteristics of the cell’s proteins, including the structural proteins, as well as the intracellular enzymes that control cytoplasmic and nuclear activities.
Nuclear Membrane
It’s two separate bilayer membranes, one inside the other.The outer membrane is continuous with the endoplasmic reticulum of the cell cytoplasm,and the space between the two nuclear membranes is also continuous with the space inside the endoplasmic reticulum.
Nucleoli and Formation of Ribosomes
The nucleolus does not have a limiting membrane. it is simply an accumulation of large amounts of RNA and proteins of the types found in ribosomes.The nucleolus becomes considerably enlarged when the cell is actively synthesizing proteins.
Formation of the nucleoli and the ribosomes in the cytoplasm outside the nucleus begins in the nucleus.
Transport of substances through the cell membrane
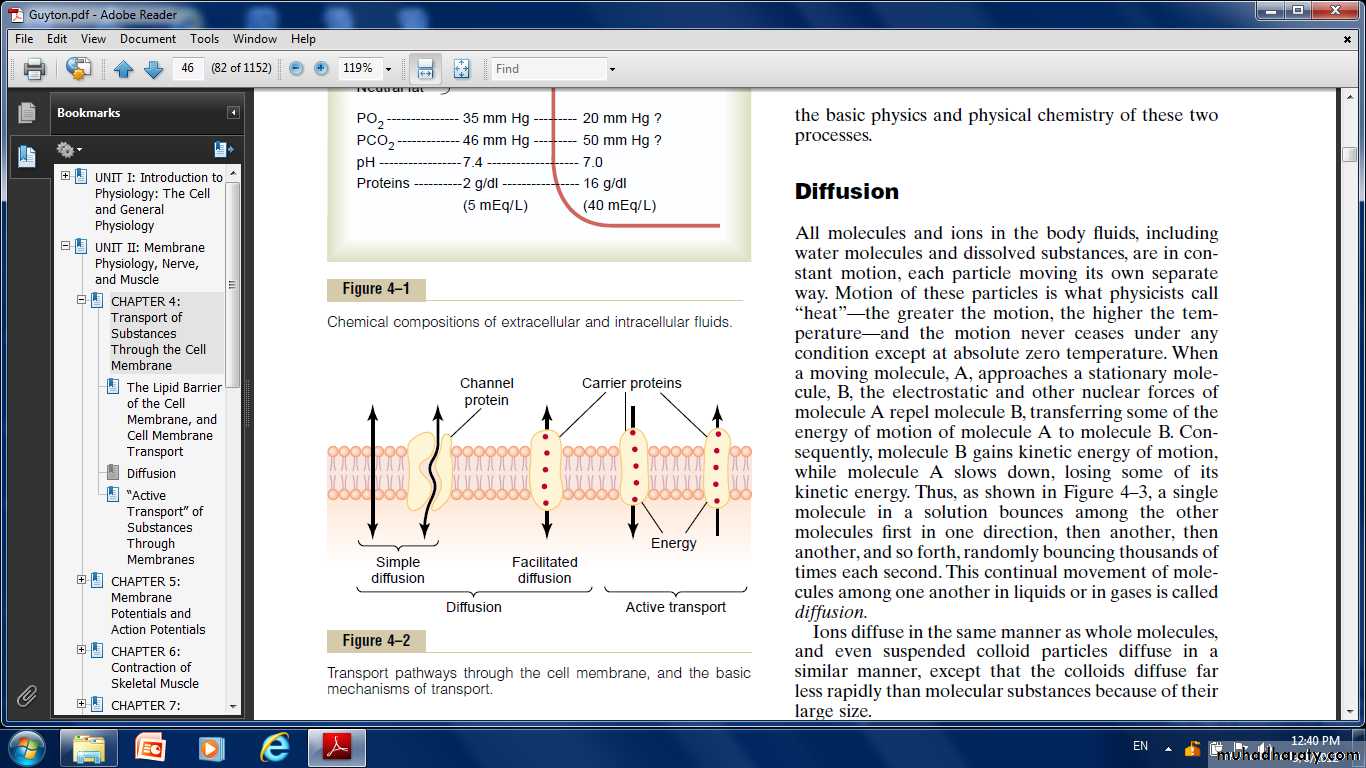
Diffusion” Versus “Active Transport.”Transport through the cell membrane, either directly through the lipid bilayer or through the proteins, occurs by one of two basic processes:
Diffusion and Active transport.
Diffusion: means random molecular movement of substances through intermolecular spaces in the membrane or in combination with a carrier protein.
Active transport: means movement of ions or other substances across the membrane in combination with a carrier protein in such a way that the carrier protein causes the substance to move from a low-concentration state to a high-concentration state.
Diffusion Through the Cell Membrane
Simple diffusion and facilitated diffusion.Simple diffusion:
Means that kinetic movement of molecules or ions occurs through a membrane opening or through intermolecular spaces without any interaction with carrier proteins in the membrane.
The rate of diffusion is determined by the amount of substance available, the velocity of kinetic motion, and the number and sizes of openings in the membrane through which the molecules or ions can move.
Facilitated diffusion:
It requires interaction of a carrier protein which aids passage of the molecules or ions through the membrane by binding chemically with them and shuttling them through the membrane.Simple diffusion can occur through the cell membrane by two pathways:
(1) through the interstices of the lipid bilayer if the diffusing substance is lipid soluble.
(2) through watery channels that penetrate all the way through the large transport proteins.
Diffusion of Lipid-Soluble Substances Through the Lipid Bilayer.
One of the most important factors that determines how rapidly a substance diffuses through the lipid bilayer is the lipid solubility of the substance. (e g. oxygen, nitrogen, carbon dioxide, and alcohol).Diffusion of Water and Other Lipid-Insoluble Molecules Through Protein Channels.
Even though water is highly insoluble in the membrane lipids, it readily passes through channels in protein molecules that penetrate all the way through the membrane.The total amount of water that diffuses in each direction through the red cell membrane during each second is about 100 times as great as the volume of the red cell itself.
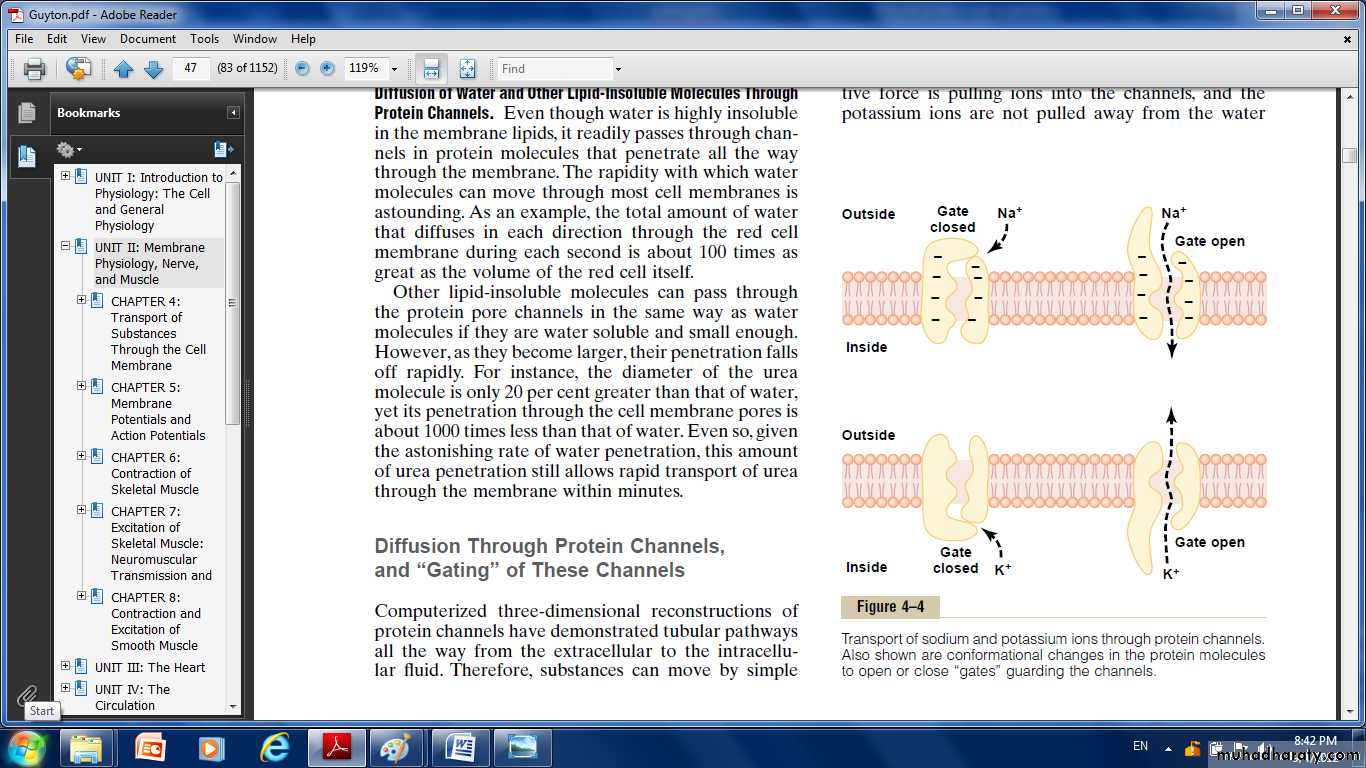
Diffusion Through Protein Channels, and “Gating” of These Channels:
The protein channels are distinguished by two important characteristics:(1) They are often selectively permeable to certain substances,
(2) Many of the channels can be opened or closed by gates.
Selective Permeability of Protein Channels.
Sodium transport channels:Its only 0.3 by 0.5 nanometer in diameter, but more important, the inner surfaces of this channel are strongly negatively charged.
These strong negative charges can pull small dehydrated sodium ions into these channels, actually pulling the sodium ions away from their hydrating water molecules.
Once in the channel, the sodium ions diffuse in either direction according to the usual laws of diffusion.
potassium transport channels:
These channels are slightly smaller than the sodium channels, only 0.3 by 0.3 nanometer, but they are not negatively charged, and their chemical bonds are different.Therefore, no strong attractive force is pulling ions into the channels, and the potassium ions are not pulled away from the water molecules that hydrate them.
The hydrated form of the potassium ion is considerably smaller than the hydrated form of sodium.
Therefore, the smaller hydrated potassium ions can pass easily through this small channel, whereas the larger hydrated sodium ions are rejected.
Gating of Protein Channels.
Gating of protein channels provides a means of controlling ion permeability of the channels.Some of the gates are actual gate like extensions of the transport protein molecule, which can close the opening of the channel or can be lifted away from the opening by a conformational change in the shape of the protein molecule itself.
The opening and closing of gates are controlled in two principals:
1. Voltage gating.The molecular conformation of the gate or of its chemical bonds responds to the electrical potential across the cell membrane.
when there is a strong negative charge on the inside of the cell membrane, this presumably could cause the outside sodium gates to remain tightly closed.
conversely, when the inside of the membrane loses its negative charge, these gates would open suddenly and allow tremendous quantities of sodium to pass inward through the sodium pores.
While the potassium gates are on the intracellular ends of the potassium channels, and they open when the inside of the cell membrane becomes positively charged.
2. Chemical gating:
Some protein channel gates are opened by the binding of a chemical substance with the protein; this causes a conformational or chemical bonding change in the protein molecule that opens or closes the gate.This is called chemical gating (eg. of chemical gating is the effect of acetylcholine on the so-called acetylcholine channel).
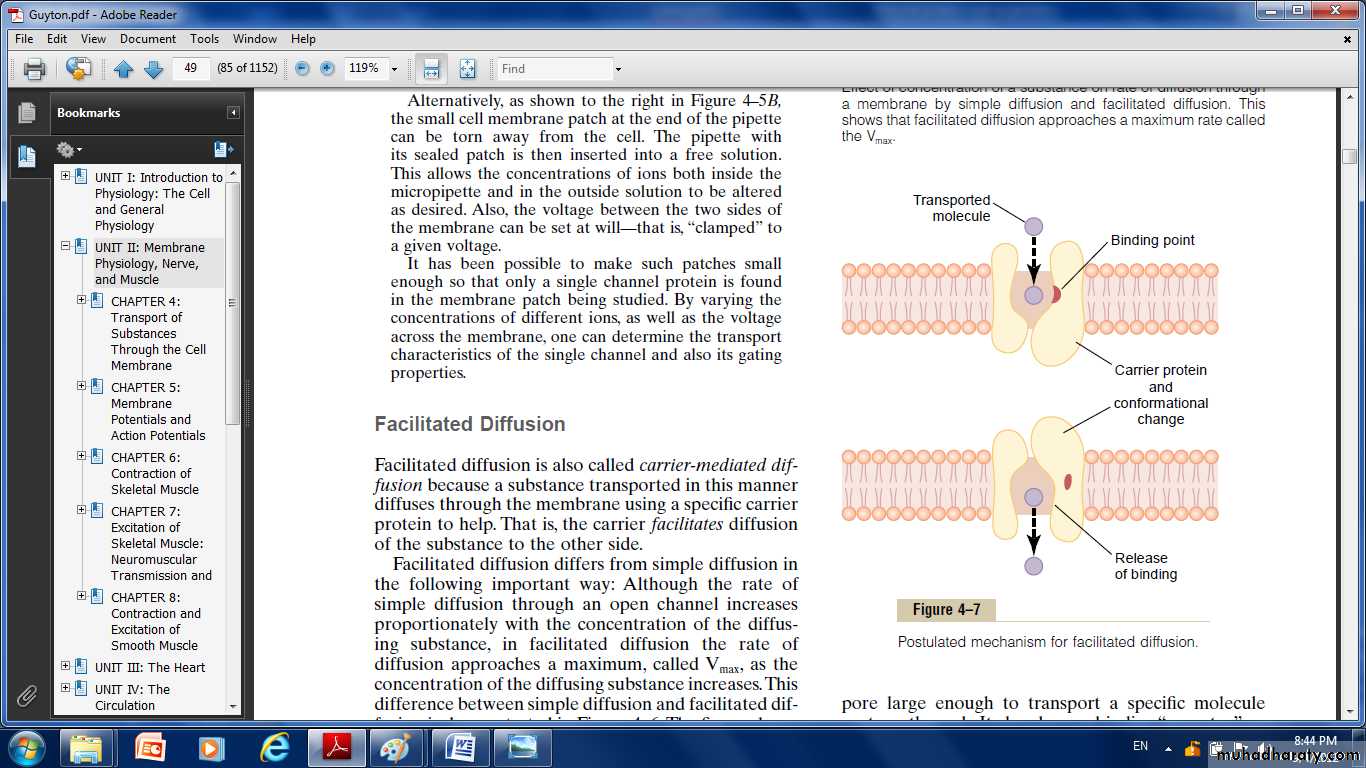
Facilitated Diffusion
Facilitated diffusion is also called carrier-mediated diffusion because a substance transported in this manner diffuses through the membrane using a specific carrier protein to help. That is, the carrier facilitates diffusion of the substance to the other side.The carrier protein with a pore large enough to transport a specific molecule through it.
There is a binding “receptor” on the inside of the protein carrier.The molecule to be transported enters the pore and becomes bound.
Then,in a fraction of a second, a conformational or chemical change occurs in the carrier protein, so that the pore now opens to the opposite side of the membrane.
Because the binding force of the receptor is weak, the thermal motion of the attached molecule causes it to break away and to be released on the opposite side of the membrane.
Among the most important substances that cross cell membranes by facilitated diffusion are glucose and most of the amino acids.
Also, insulin can increase the rate of facilitated diffusion of glucose as much as 10-fold to 20-fold.
This is the principal mechanism by which insulin controls glucose use in the body.
Factors That Affect Net Rate of Diffusion:
• Concentration Difference :The rate at which the substance diffuses inward is proportional to the concentration of molecules on the outside, because this concentration determines how many molecules strike the outside of the membrane each second.
Conversely, the rate at which molecules diffuse outward is proportional to their concentration inside the membrane. Net diffusion μ (Co - Ci) in which (Co) is concentration outside and (Ci ) is concentration inside.
• 2. Membrane Electrical Potential.
• If an electrical potential is applied across the membrane, the electrical charges of the ions cause them to move through the membrane even though no concentration difference exists to cause movement.3. Effect of a Pressure Difference Across the Membrane.
Considerable pressure difference develops between the two sides of a diffusible membrane.This occurs, for instance, at the blood capillary membrane in all tissues of the body.
The pressure is about 20 mm Hg greater inside the capillary than outside.
“Active Transport” of Substances Through Membranes:
When a cell membrane moves molecules or ions against a concentration gradient or against an electrical or pressure gradient the process is called active transport.Alarge concentration of a substance is required in the intracellular fluid even though the extracellular fluid contains only a small concentration. This is true, for instance, for potassium ions.
Conversely, it is important to keep the concentrations of other ions very low inside the cell even though their concentrations in the extracellular fluid are great. This is especially true for sodium ions.
Different substances that are actively transported through at least some cell membranes include sodium ions, potassium ions, calcium ions, iron ions, hydrogen ions, chloride ions, iodide ions, urate ions, several different sugars, and most of the amino acids.
Primary Active Transport Sodium-Potassium Pump:
Sodium-potassium (Na+-K+) pump, it pumps sodium ions outward through the cell membrane of all cells and at the same time pumps potassium ions from the outside to the inside.
This pump is responsible for maintaining the sodium and potassium concentration differences across the cell membrane, as well as for establishing a negative electrical voltage inside the cells.
The carrior protein has the following characters :
1. It has three receptor sites for binding sodium ions on the portion of the protein that protrudes to the inside of the cell.2. It has two receptor sites for potassium ions on the outside.
3. The inside portion of this protein near the sodium binding sites has ATPase activity.
When two potassium ions bind on the outside of the carrier protein and three sodium ions bind on the inside, the ATPase function of the protein becomes activated. This then cleaves one molecule of ATP, splitting it to adenosine diphosphate (ADP) and liberating a high-energy phosphate bond of energy.
This liberated energy is then believed to cause a chemical and conformational change in the protein carrier molecule, extruding the three sodium ions to the outside and the two potassium ions to the inside.
Importance of the Na+-K+ Pump for Controlling Cell Volume.
One of the most important functions of the Na+-K+ pump is to control the volume of each cell.Without function of this pump, most cells of the body would swell until they burst.
The mechanism for controlling the volume is as follows:
Inside the cell are large numbers of proteins and other organic molecules that cannot escape from the cell. Most of these are negatively charged and therefore attract large numbers of potassium, sodium, and other positive ions as well.
All these molecules and ions then cause osmosis of water to the interior of the cell.
Unless this is checked, the cell will swell indefinitely until it bursts. The normal mechanism for preventing this; is the Na+-K+ pump.This device pumps three Na+ ions to the outside of the cell for every two K+ ions pumped to the interior. Also, the membrane is far less permeable to sodium ions than to potassium ions, so that once the sodium ions are on the outside, they have a strong tendency to stay there.
Thus, this represents a net loss of ions out of the cell, which initiates osmosis of water out of the cell as well.
If a cell begins to swell for any reason, this automatically activates the Na+-K+ pump, moving still more ions to the exterior and carrying water with them.
Therefore, the Na+-K+ pump performs a continual surveillance role in maintaining normal cell volume.
Electrogenic Nature of the Na+-K+ Pump.
The fact that the Na+-K+ pump moves three Na+ ions to the exterior for every two K+ ions to the interior means that a net of one positive charge is moved from the interior of the cell to the exterior for each cycle of the pump.This creates positivity outside the cell but leaves a deficit of positive ions inside the cell; that is, it causes negativity on the inside.
Primary Active Transport of Calcium Ions
Another important primary active transport mechanism is the calcium pump. Calcium ions are normally maintained at extremely low concentration in the intracellular cytosol of virtually all cells in the body, at a concentration about 10,000 times less than that in the extracellular fluid.This is achieved mainly by two primary active transport calcium pumps. One is in the cell membrane and pumps calcium to the outside of the cell.
The other pumps calcium ions into one or more of the intracellular vesicular organelles of the cell, such as the sarcoplasmic reticulum of muscle cells and the mitochondria in all cells.
In each of these instances, the carrier protein penetrates the membrane and functions as an enzyme ATPase, having the same capability to cleave ATP as the ATPase of the sodium carrier protein.
Primary Active Transport of Hydrogen Ions At two places in the body, primary active transport of hydrogen ions is very important:
In the gastric glands of the stomach.
In the late distal tubules and cortical collecting ducts of the kidneys.
Secondary Active Transport— Co-Transport and Counter Transport
When sodium ions are transported out of cells by primary active transport, a large concentration gradient of sodium ions across the cell membrane usually develops.This gradient represents a storehouse of energy because the excess sodium outside the cell membrane is always attempting to diffuse to the interior.
Under appropriate conditions, this diffusion energy of sodium can pull other substances along with the sodium through the cell membrane.
This phenomenon is called co-transport; it is one form of secondary active transport.
For sodium to pull another substance along with it, a coupling mechanism is required. This is achieved by means of still another carrier protein in the cell membrane.
The carrier in this instance serves as an attachment point for both the sodium ion and the substance to be co-transported.
Once they both are attached, the energy gradient of the sodium ion causes both the sodium ion and the other substance to be transported together to the interior of the cell.
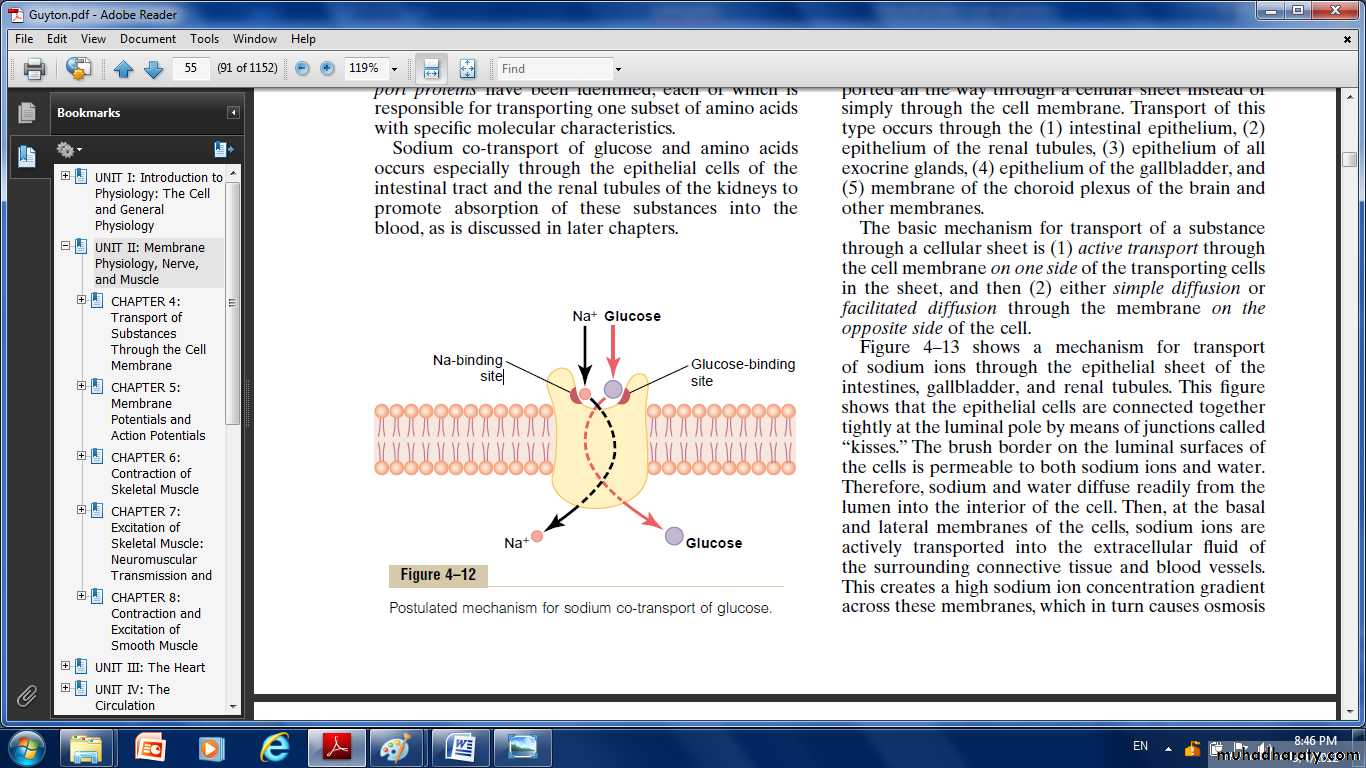
Co-Transport of Glucose and Amino Acids:
Along with Sodium Ions Glucose and many amino acids are transported into most cells against large concentration gradients; the mechanism of this is entirely by co-transport, the transport carrier protein has two binding sites on its exterior side, one for sodium and one for glucose. Also, the concentration of sodium ions is very high on the outside and very low inside, which provides energy for the transport.When they both become attached, the conformational change takes place automatically, and the sodium and glucose are transported to the inside of the cell at the same time.
Sodium co-transport of the amino acids occurs in the same manner as for glucose.
Counter-transport:
sodium ions again attempt to diffuse to the interior of the cell because of their large concentration gradient.However, the substance to be transported is on the inside of the cell and must be transported to the outside.
Therefore, the sodium ion binds to the carrier protein where it projects to the exterior surface of the membrane.
while the substance to be counter-transported binds to the interior projection of the carrier protein.
Once both have bound, a conformational change occurs, and energy released by the sodium ion moving to the interior causes the other substance to move to the exterior.
Sodium Counter-Transport of Calcium.
Sodium-calcium counter-transport occurs through all or almost all cell membranes, with sodium ions moving to the interior and calcium ions to the exterior.
Both bound to the same transport protein in a counter transport mode.
Sodium-hydrogen counter-transport:
Occurs in several tissues, in the proximal tubules of the kidneys, where sodium ions move from the lumen of the tubule to the interior of the tubular cell, while hydrogen ions are counter transported into the tubule lumen. So it can transport extremely large numbers of hydrogen ions, thus making it a key to hydrogen ion control in the body fluids.Active Transport Through Cellular Sheets:
At many places in the body, substances must be transported all the way through a cellular sheet instead of simply through the cell membrane. Transport of this type occurs through the:intestinal epithelium.
epithelium of the renal tubules.
epithelium of the gallbladder.
membrane of the choroid plexus of the brain.
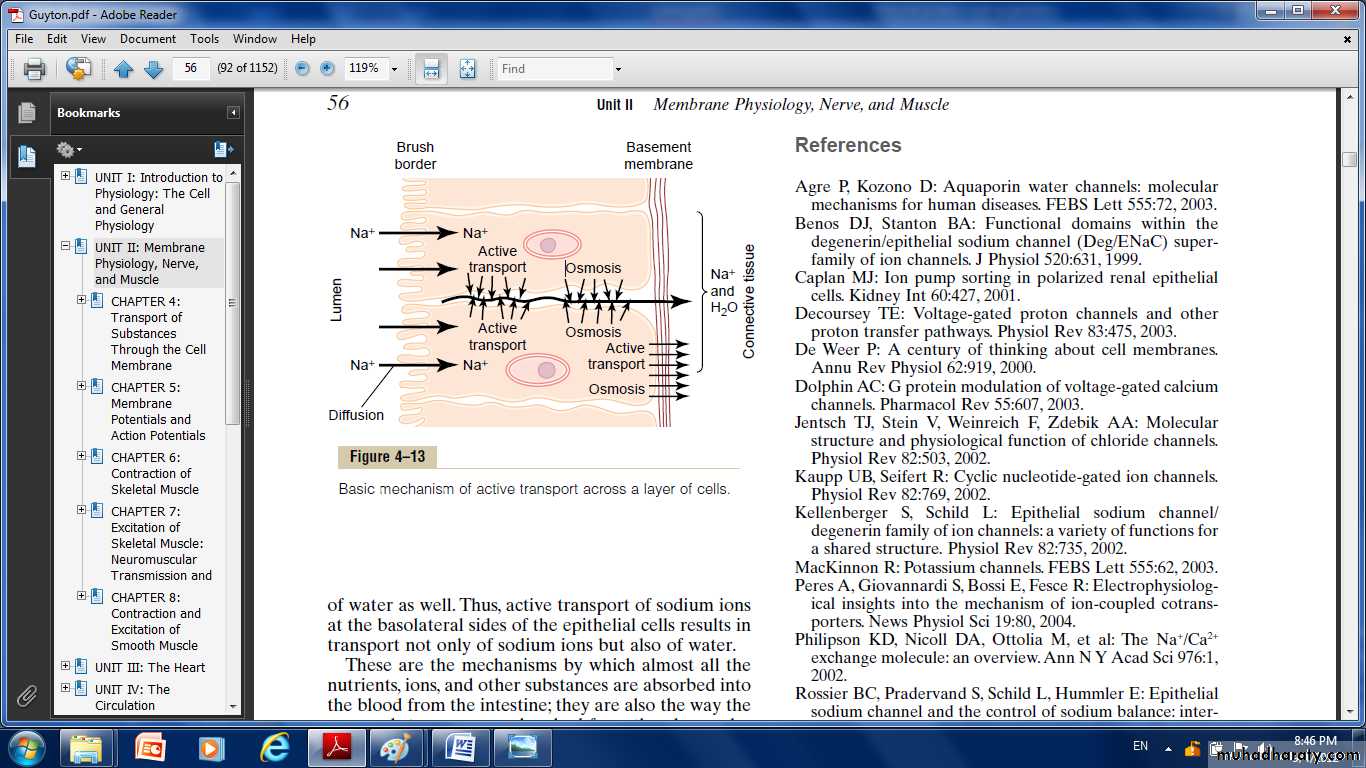
The basic mechanism for transport of a substance through a cellular sheet is:
(1) active transport through the cell membrane on one side of the transporting cells in the sheet.(2) either simple diffusion or facilitated diffusion through the membrane on the opposite side of the cell.
The brush border on the luminal surfaces of the cells is permeable to both sodium ions and water. Therefore, sodium and water diffuse readily from the lumen into the interior of the cell.
Then, at the basal and lateral membranes of the cells, sodium ions are actively transported into the extracellular fluid of the surrounding connective tissue and blood vessels.
This creates a high sodium ion concentration gradient across these membranes, which in turn causes osmosis of water as well.
Thus, active transport of sodium ions at the basolateral sides of the epithelial cells results in transport not only of sodium ions but also of water.
These are the mechanisms by which almost all the nutrients, ions, and other substances are absorbed into the blood from the intestine.
They are also the way the same substances are reabsorbed from the glomerular filtrate by the renal tubules.
The Body Fluid Compartments:
Extracellular and Intracellular Fluids;Interstitial Fluid and Edema
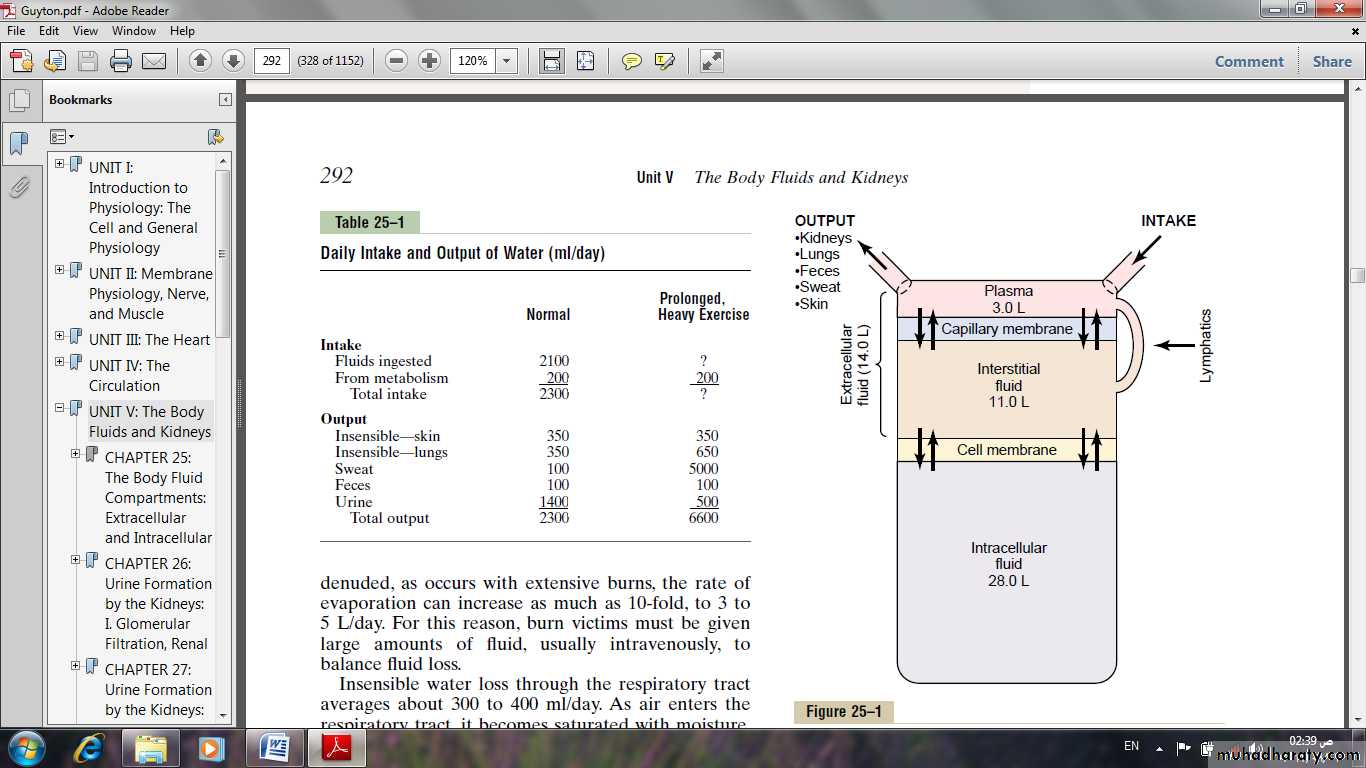
Daily Intake of Water
Water is added to the body by two major sources:(1) It is ingested in the form of liquids or water in the food, which together normally add about 2100 ml/day to the body fluids.
(2) It is synthesized in the body as a result of oxidation of carbohydrates, adding about 200 ml/day. This provides a total water intake of about 2300 ml/day.
Daily Loss of Body Water
Insensible Water Loss; there is a continuous loss of water by evaporation from the respiratory tract and diffusion through the skin, which together account for about 700 ml/day of water loss under normal conditions. This is termed insensible water loss because we are not consciously aware of it.
The insensible water loss through the skin occurs independently of sweating, the average water loss by diffusion through the skin is about 300 to 400 ml/day.
Fluid Loss in Sweat.
The amount of water lost by sweating is highly variable, depending on physical activity and environmental temperature. The volume of sweat normally is about 100 ml/day, but in very hot weather or during heavy exercise, water loss in sweat occasionally increases to 1 to 2 L/hour.Water Loss in Feces.
Only a small amount of water (100 ml/day) normally is lost in the feces. This can increase to several liters a day in people with severe diarrhea.
Water Loss by the Kidneys.
The remaining water loss from the body occurs in the urine excreted by the kidneys.the most important means by which the body maintains a balance between water and electrolytes intake and output in the body, is by controlling the rates at which the kidneys excrete these substances.
urine volume can be as low as 0.5 L/day in a dehydrated person or as high as 20 L/day in a person who has been drinking tremendous amounts of water.
Body Fluid Compartments
the extracellular fluid .the intracellular fluid.
The extracellular fluid:
Is divided into the interstitial fluid and the blood plasma.
In the average 70-kilogram adult human, the total body water is about 60% of the body weight, or about 42 liters.
This percentage can change, depending on age, gender, and degree of obesity.
Intracellular Fluid Compartment
About 28 of the 42 liters (40% of the total body weight ) of fluid in the body are inside the 75 trillion cells and are collectively called the intracellular fluid.The intracellular fluid of all the different cells together is considered to be one large fluid compartment.
Extracellular Fluid Compartment
All the fluids outside the cells are collectively called the extracellular fluid.Together these fluids account for about 20% of the body weight (14 liters).
The two largest compartments of the extracellular fluid are:
The interstitial fluid, which makes up more than three fourths of the extracellular fluid.
The plasma, which makes up almost one fourth of the extracellular fluid, or about 3 liters.
The plasma is the non cellular part of the blood; it exchanges substances continuously with the interstitial fluid through the pores of the capillary membranes.
These pores are highly permeable to almost all solutes in the extracellular fluid except the proteins.
Therefore, the extracellular fluids are constantly mixing, so that the plasma and interstitial fluids have about the same composition except for proteins, which have a higher concentration in the plasma.
Blood Volume
Blood contains both extracellular fluid (the fluid in plasma) and intracellular fluid (the fluid in the red blood cells).Blood is considered to be a separate fluid compartment because it is contained in a chamber of its own, the circulatory system.
The blood volume is especially important in the control of cardiovascular dynamics.
The average blood volume of adults is about 7% of body weight, or about 5 liters.About 60% of the blood is plasma and 40% is red blood cells, but these percentages can vary considerably in different people, depending on gender, weight, and other factors.
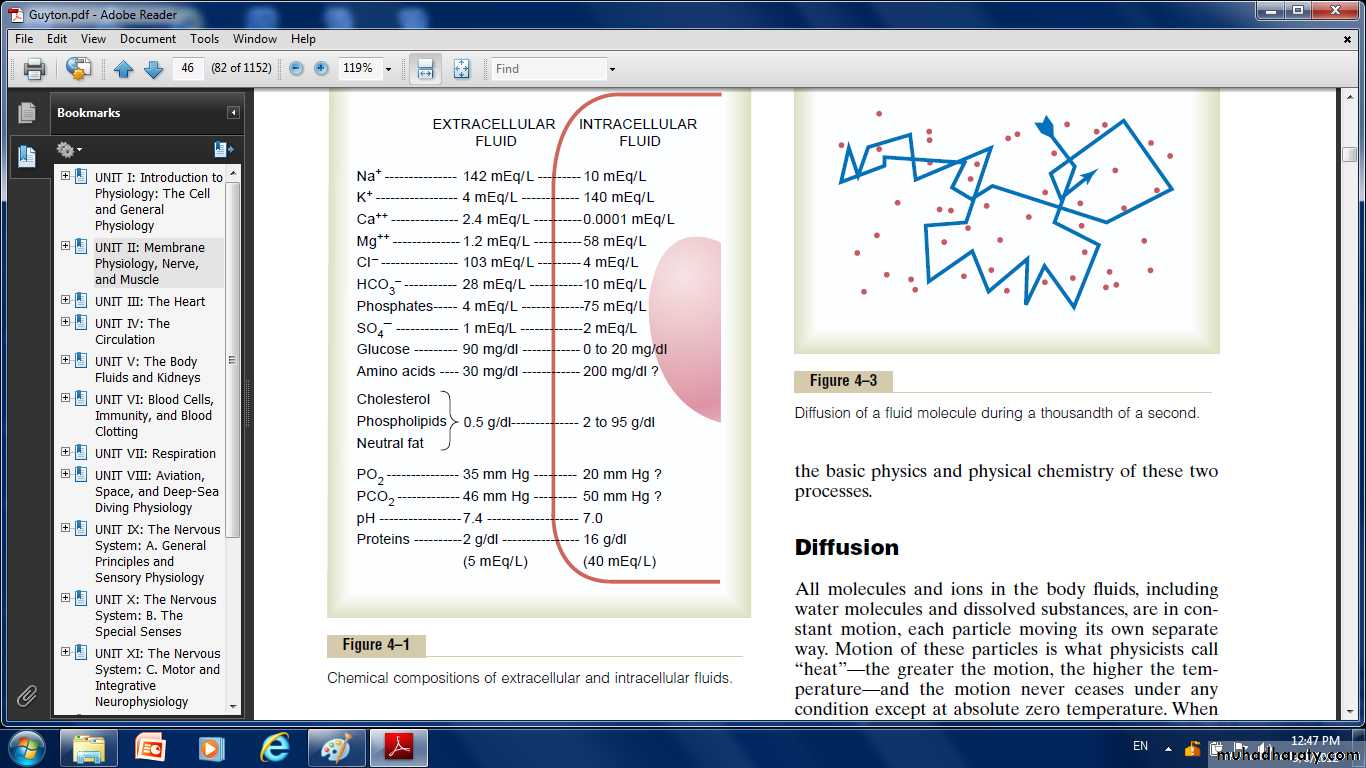
Ionic Composition of Plasma and Interstitial Fluid Is Similar
Because the plasma and interstitial fluid are separated only by highly permeable capillary membranes, their ionic composition is similar.The most important difference between these two compartments is the higher concentration of protein in the plasma; because the capillaries have a low permeability to the plasma proteins, only small amounts of proteins are leaked into the interstitial spaces in most tissues.
The Donnan effect:
The concentration of positively charged ions (cations) is slightly greater (about 2%) in the plasma than in the interstitial fluid.The plasma proteins have a net negative charge and, therefore, tend to bind cations, such as sodium and potassium ions, thus holding extra amounts of these cations in the plasma along with the plasma proteins.
Conversely, negatively charged ions (anions) tend to have a slightly higher concentration in the interstitial fluid compared with the plasma, because the negative charges of the plasma proteins repel the negatively charged anions.
The concentration of ions in interstitial fluid and in the plasma is considered to be about equal.
The extracellular fluid, including the plasma and the interstitial fluid, contains large amounts of sodium, chloride and bicarbonate ions, but only small quantities of potassium, calcium, magnesium, phosphate, and organic acid ions.
Important Constituents of the Intracellular Fluid
the intracellular fluid contains only small quantities of sodium and chloride ions and almost no calcium ions.it contains large amounts of potassium and phosphate ions plus moderate quantities of magnesium and sulfate ions, all of which have low concentrations in the extracellular fluid.
Also, cells contain large amounts of protein, almost four times as much as in the plasma.
Regulation of Fluid Exchange and Osmotic Equilibrium Between Intracellular and Extracellular Fluid:
The relative amounts of extracellular fluid distributed between the plasma and interstitial spaces are determined mainly by the balance of hydrostatic and colloid osmotic forces across the capillary membranes.
The distribution of fluid between intracellular and extracellular compartments is determined mainly by the osmotic effect of the smaller solutes— especially sodium, chloride, and other electrolytes—acting across the cell membrane.
The reason for this is that the cell membranes are highly permeable to water but relatively impermeable to even small ions such as sodium and chloride.
Therefore, water moves across the cell membrane rapidly, so that the intracellular fluid remains isotonic with the extracellular fluid.
Basic Principles of Osmosis and Osmotic Pressure
Osmosis is the net diffusion of water across a selectively permeable membrane from a region of high water concentration to one that has a lower water concentration.When a solute is added to pure water, this reduces the concentration of water in the mixture. Thus, the higher the solute concentration in a solution, the lower the water concentration.
So , water diffuses from a region of low solute concentration (high water concentration) to one with a high solute concentration (low water concentration).
Thus, if a solute such as sodium chloride is added to the extracellular fluid, water rapidly diffuses from the cells through the cell membranes into the extracellular fluid until the water concentration on both sides of the membrane becomes equal.
Conversely, if a solute such as sodium chloride is removed from the extracellular fluid, water diffuses from the extracellular fluid through the cell membranes into the cells.
The rate of diffusion of water is called the rate of osmosis.
The osmotic pressure of a solution is directly proportional to the concentration of osmotically active particles in that solution.
Regardless of whether the solute is a large molecule or a small molecule. (eg. one molecule of albumin with a molecular weight of 70,000 has the same osmotic effect as one molecule of glucose with a molecular weight of 180.
One molecule of sodium chloride, however, has two osmotically active particles, Na+ and Cl–, and therefore has twice the osmotic effect of either an albumin molecule or a glucose molecule.
Osmolarity of the Body Fluids.
There are an approximate osmolarity of the various osmotically active substances in plasma, interstitial fluid, and intracellular fluid.About 80 per cent of the total osmolarity of the interstitial fluid and plasma is due to sodium and chloride ions.
whereas for intracellular fluid; Almost half the osmolarity is due to potassium ions, and the remainder is divided among many other intracellular substances.
The total osmolarity of each of the three compartments is about 300 mOsm/L, with the plasma being about 1 mOsm/L greater than that of the interstitial and intracellular fluids.
The slight difference between plasma and interstitial fluid is caused by the osmotic effects of the plasma proteins, which maintain about 20 mm Hg greater pressure in the capillaries than in the surrounding interstitial spaces.
Isotonic, Hypotonic, and Hypertonic Fluids.
If a cell is placed in a solution of impermeant solutes having an osmolarity of 282 mOsm/L, the cells will not shrink or swell because the water concentration in the intracellular and extracellular fluids is equal and the solutes cannot enter or leave the cell.Such a solution is said to be isotonic because it neither shrinks nor swells the cells.
Examples of isotonic solutions include a 0.9% solution of sodium chloride or a 5% glucose solution.
These solutions are important in clinical medicine because they can be infused into the blood without the danger of upsetting osmotic equilibrium between the intracellular and extracellular fluids.
If a cell is placed into a hypotonic solution that has a lower concentration of impermeant solutes (less than 282 mOsm/L).
Water will diffuse into the cell, causing it to swell; water will continue to diffuse into the cell, diluting the intracellular fluid while also concentrating the extracellular fluid until both solutions have about the same osmolarity.
Solutions of sodium chloride with a concentration of less than 0.9% are hypotonic and cause cells to swell.
If a cell is placed in a hypertonic solution having a higher concentration of impermeant solutes, water will flow out of the cell into the extracellular fluid, concentrating the intracellular fluid and diluting the extracellular fluid.
In this case, the cell will shrink until the two concentrations become equal.
Sodium chloride solutions of greater than 0.9% are hypertonic.
Edema: Excess Fluid in the Tissues
Edema refers to the presence of excess fluid in the body tissues; edema occurs mainly in the extracellular fluid compartment, but it can involve intracellular fluid as well.Intracellular Edema
Two conditions are especially prone to cause intracellular swelling:
(1) Depression of the metabolic systems of the tissues.
(2) Lack of adequate nutrition to the cells.
For example, when blood flow to a tissue is decreased, the delivery of oxygen and nutrients is reduced so the normal tissue metabolism is reduced also, so, the cell membrane ionic pumps become depressed.
When this occurs, sodium ions that normally leak into the interior of the cell can no longer be pumped out of the cells, and the excess sodium ions inside the cells cause osmosis of water into the cells.
Sometimes this can increase intracellular volume of a tissue area (in entire ischemic leg) two to three times normal.
Extracellular Edema
Extracellular fluid edema occurs when there is excess fluid accumulation in the extracellular spaces. There are two general causes of extracellular edema:(1) Abnormal leakage of fluid from the plasma to the interstitial spaces across the capillaries.
(2) Failure of the lymphatic to return fluid from the interstitium back into the blood.
Fluids in the “Potential Spaces” of the Body
pleural cavity, Pericardial cavity, peritoneal cavity, and synovial cavities. Virtually all these potential spaces have surfaces that almost touch each other, with only a thin layer of fluid in between, and the surfaces slide over each other. To facilitate the sliding, a viscous proteinaceous fluid lubricates the surfaces.Edema Fluid in the Potential Spaces Is Called “Effusion.”
When edema occurs in the subcutaneous tissues adjacent to the potential space, this fluid is called effusion.Thus, lymph blockage or any of the multiple abnormalities that can cause excessive capillary filtration can cause effusion in the same way that interstitial edema is caused.
The abdominal cavity is especially prone to collect effusion fluid, and in this instance, the effusion is called ascites. In serious cases, 20 liters or more of ascitic fluid can accumulate.