.
It is abnormal deposition of calcium salts, together with smaller amounts of iron, magnesium, and other minerals in any tissue except bones and teeth.
There are two distinct types of Pathological calcification:-
1- Dystrophic calcification
Characterized by deposition of calcium salts in dead or degenerated tissues with normal calcium metabolism and normal serum calcium levels.
Dystrophic calcification is encountered in areas of necrosis of any type. It is visible in advanced atherosclerosis, associated with intimal injury in the aorta and large arteries and characterized by accumulation of lipids. Although dystrophic calcification may be an incidental finding indicating past cell injury, it may also be a cause of organ dysfunction. For example, calcification can develop in aging or damaged heart valves, resulting in severely compromised valve motion. Dystrophic calcification of the aortic valves is an important cause of aortic stenosis in the elderly.
1
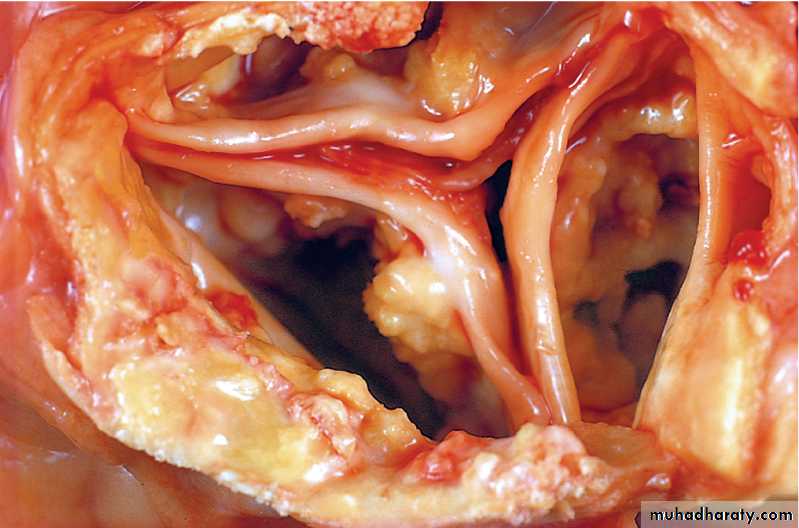
Calcification of the aortic valve. the unopened aortic valve in a heart with calcific aortic stenosis. The semilunar cusps are thickened and fibrotic. Behind each cusp are large, irregular masses of dystrophic calcification that will prevent normal opening of the cusps
2
pathogenesis of dystrophic calcification
The pathogenesis of dystrophic calcification involves initiation (or nucleation) and propagation, both of which may be either intracellular or extracellular the ultimate end product is the formation of crystalline calcium phosphate which formed by reaction of phosphate ions with calcium ions.Its involves two steps:-
1-In initiation (or nucleation)
- Initiation in extracellular sites occurs in membrane-bound vesicles (in normal cartilage and bone). calcium is initially concentrated in these vesicles by its affinity for membrane phospholipids, while phosphates accumulate as a result of the action of membrane-bound phosphatases.
- Initiation of intracellular calcification occurs in the mitochondria of dead cells that have lost their ability to regulate intracellular calcium.
2- propagation
After initiation in either location, propagation of crystal formation occurs.
3
2- Metastatic calcification
Characterized by deposition of calcium salts in normal tissues due to derangement in calcium metabolism (hypercalcemia)
The four major causes of hypercalcemia are
(1) increased secretion of parathyroid hormone, due to either primary parathyroid tumors or production of parathyroid hormone-related protein by other malignant tumors.
(2) destruction of bone due to the effects of accelerated turnover (e.g., Paget disease), immobilization, or tumors (increased bone catabolism associated with multiple myeloma, leukemia).
(3) vitamin D-related disorders including vitamin D intoxication and sarcoidosis
(4) renal failure, in which phosphate retention leads to secondary hyperparathyroidism
4
Cellular aging
Cellular aging is the result of decrease in the proliferative capacity and life span of cells and the effects of continuous exposure to exogenous factors that cause cellular and molecular damage. The aging is controlled by particular genes has spurred enormous interest in its molecular pathways and in devising ways to manipulate a process that was once considered inexorable.5
حفزت جينات معينة اهتمامًا كبيرًا في مساراتها الجزيئية وفي استنباط طرق لمعالجة عملية كانت تعتبر ذات يوم لا تطاق
6
Several mechanisms are known or suspected to be responsible for cellular aging:-
1- DNA damage. Cellular aging is associated with increasing DNA damage, which may happen during normal DNAreplication and can be enhanced by free radicals.
Although most DNA damage is repaired by DNA repair enzymes, some persists and accumulates and cause cells aging.
2- Decreased cellular replication. All normal cells have a limited capacity for replication, and after a fixed number of divisions cells become arrested in a terminally nondividing state, known as replicative senescence. Aging is associated with progressive replicative senescence of cells i.e cells from children have the capacity to undergo more rounds of replication than do cells from older people.
7
3- Reduced regenerative capacity of tissue stem cells. Recent studies suggest that with age, the p16 (CDKN2A) protein accumulates in stem cells, and they progressively lose their capacity to self-renew. p16 is a physiological inhibitor of cell regeneration .
4- Accumulation of metabolic damage. Cellular life span is also determined by a balance between damage resulting from accumulation of metabolits occurring within the cell and counteracting molecular responses that can repair the damage. One group of potentially toxic products of normal metabolism is reactive oxygen species. these byproducts of oxidative phosphorylation cause modifications of proteins, lipids, and nucleic acids.
8
Inflammation
Inflammation is a protective response intended to eliminate the initial cause of cell injury as well as the necrotic cells and tissues resulting from the original insult.Inflammation accomplishes its protective mission by diluting, destroying, or neutralizing harmful agents (e.g., microbes and toxins) and then followed by the events that eventually heal and repair the sites of injury . Without inflammation, infections would go unchecked and wounds would never heal.
9
Causes of inflammation
1- Infective agents such as bacteria ,viruses and their toxins ,fungi, parasites.2-immunological agents as cell mediated and antigen-antibody reaction.
3-physical agents like heat ,cold ,radiation, and mechanical trauma.
4-Chemical agents like organic and in organic poisons.
5-Inert material as foreign bodies.
10
Cardinal signs of inflammation
1- Rubor (redness).
2-Tumor(swelling).
3-Calor(heat).
4-Dolor(pain).
5-Functiolaesa(loss of function).
11
Types of inflammation
Inflammation may be acute or chronic depended on:-1-the nature of the stimuli.
2-the effectiveness of the initial inflammatory reaction in eliminating the stimulus or damaged tissues.
Acute inflammation is rapid in onset and short in duration, lasting from a few minutes to as long as a few days, and is characterized by fluid and plasma protein exudation and a predominantly neutrophilic leukocyte accumulation.
Chronic inflammation may be more insidious, and longer duration (days to years), and is characterized by influx of lymphocytes and macrophages with vascular proliferation and fibrosis (scarring).
12
Acute inflammation
Acute inflammation is a rapid response to injury or microbes and other foreign substances .its is designed to deliver leukocytes and plasma proteins to sites of injury. And then the leukocytes clear the invaders and begin the process of digesting and getting rid of necrotic tissues.Stimuli for Acute Inflammation
Acute inflammatory reactions may be triggered by a variety of stimuli.
1-Infections (bacterial, viral, fungal, parasitic) are among the most common and medically important causes of inflammation.
2-Trauma (blunt and penetrating) and physical and chemical agents (, e.g., burns or frostbite; irradiation; some environmental chemicals) injure host cells and elicit inflammatory reactions.
3-Tissue necrosis (from any cause), including ischemia (as in a myocardial infarct) and physical and chemical injury.
4-Foreign bodies (splinters, dirt, sutures).
5-Immune reactions (also called hypersensitivity reactions) against environmental substances or against self tissues.
13
Acute inflammation has two major components:-
1- Vascular changes: alterations in vessel caliber resulting in increased blood flow (vasodilation) and structural changes that permit plasma proteins to leave the circulation (increased vascular permeability).2- Cellular events: emigration of the leukocytes from the microcirculation and accumulation in the site of injury (cellular recruitment and activation). The principal leukocytes in acute inflammation are neutrophils (polymorphonuclear leukocytes).
14
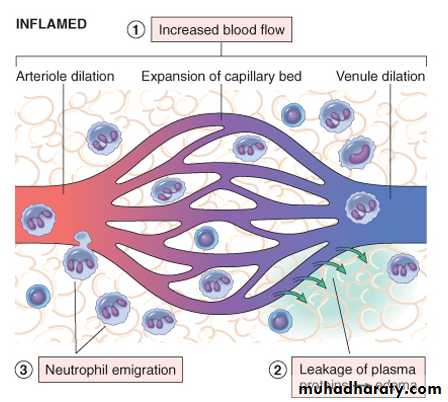
The major local manifestations of acute inflammation, compared to normal. (1) Vascular dilation and increased blood flow (causing erythema and warmth), (2) extravasation and deposition of plasma fluid and proteins (edema), and (3) leukocyte (mainly neutrophil) emigration and accumulation in the site of injury.
15
Vascular Changes
A- Changes in Vascular Caliber and Flow- After transient vasoconstriction (lasting only for seconds) .
- Arteriolar vasodilation occurs, resulting in locally increased blood flow and engorgement of the down-stream capillary beds. This vascular expansion is the cause of the redness (erythema) and warmth characteristically seen in acute inflammation.
- protein-rich fluid moves into the extravascular tissues. This causes the red blood cells to become more concentrated, then increasing blood viscosity and slowing the circulation.
- These changes are reflected microscopically by numerous dilated small vessels packed with erythrocytes and slowly flowing blood, a process called stasis. As stasis develops, leukocytes (principally neutrophils) begin to accumulate along the vascular endothelial surface, a process called margination.
16
B- Increased Vascular Permeability
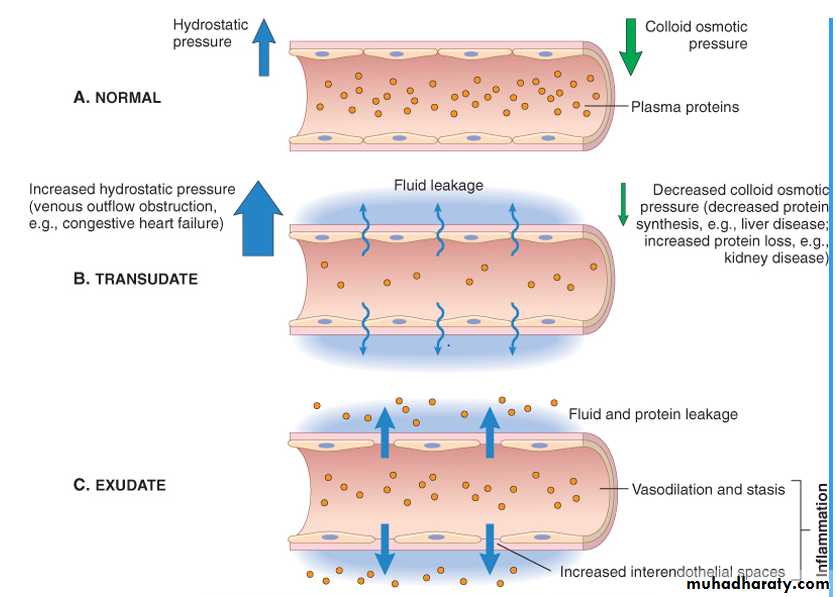
- transudate arteriolar vasodilation and increased volume of blood flow lead to a rise in intravascular hydrostatic pressure, resulting in movement of fluid from capillaries into the tissues. This fluid, is blood plasma and contains little protein .- an exudates is movement of protein-rich fluid and even cells into the interstitium due to increasing vascular permeability .
- Edema; Accumulation of fluid in extravascular spaces. this fluid may be a transudate or exudate.
-Pus :- purulent exudates is an inflammatory exudate rich in leukocytes (mostly neutrophils) , the debris of dead cells and microbes.
17
Formation of transudates and exudates A, Normal hydrostatic pressure (blue arrows). B, A transudate is formed when fluid leaks out because of increased hydrostatic pressure or decreased osmotic pressure. C, An exudate is formed in inflammation because vascular permeability increases as a result of increased interendothelial spaces.
18
mechanisms may contribute to increased vascular permeability in acute inflammatory reactions.
1- Endothelial cell contraction leading to intercellular gaps is the most common cause of increased vascular permeability. It is a reversible process elicited by histamine, bradykinin, leukotrienes, and many other chemical mediators.
2- Leukocyte-mediated endothelial injury: leukocyte accumulation along the vessel wall. activated leukocytes release many toxic mediators that may cause endothelial injury or detachment.
3- Increased transport o f proteins and fluid called transcytosis.
19