Tissue Repair: Regeneration, Healing, and Fibrosis
Repair refers to the restoration of tissue architecture and function after an injury.It occurs by two types of reactions:-
1- Regeneration:- the process in which Some tissues are able to replace the damaged components and return to a normal state.
2- scar formation:- the process which occur If the injured tissues are incapable of complete restitution, or if the supporting structures of the tissue are severely damaged, repair occurs by laying down of connective (fibrous) tissue.
fibrosis is most often used to describe the extensive deposition of collagen fibers that occurs in the lungs, liver, kidney, and other organs as a consequence of chronic inflammation, or in the myocardium after extensive ischemic necrosis (infarction).
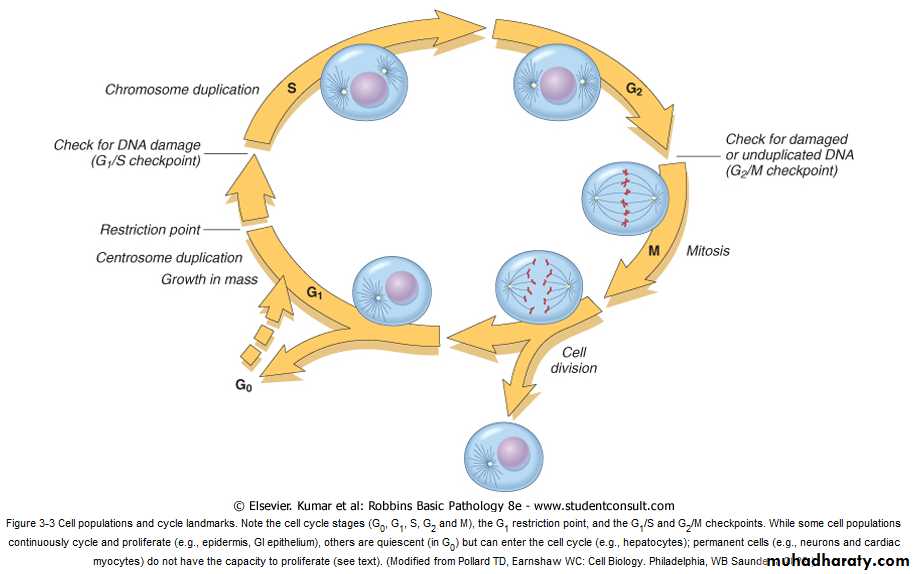
The Cell Cycle
The sequence of events that control the proliferation of cells are DNA replication and mitosis.the cell cycle has multiple controls, both positive and negative.
The cycle consists of:-
1- the presynthetic growth phase 1 (G1).
2-the DNA synthesis phase (S).
3-the premitotic growth phase 2 (G2).
4- the mitotic phase (M).
Non-dividing cells are either in cell cycle arrest in G1 or they exit the cycle to enter a phase called G0. Any stimulus that initiates cell proliferation, such as exposure to growth factors, needs to promote the G0/G1 transition and the entry of cells into the first, i.e. G1, phase of the cycle.
Checkpoint controls prevent DNA replication or mitosis of damaged cells and either transiently stop the cell cycle to allow for DNA repair or eliminate irreversibly damaged cells by apoptosis. Progression through the cell cycle from G1 is regulated by proteins called cyclins, which form complexes with enzymes called cyclin-dependent kinases (CDKs). CDKs work by promoting DNA replication and various aspects of the mitotic process and are required for cell cycle progression.
Proliferative Capacities of Tissues
The ability of tissues to repair themselves is influenced by their intrinsic proliferative capacity. Based on this criterion, the tissues of the body are divided into three groups.1- Continuously Dividing Tissues (labile tissues)
Cells of these tissues are continuously being lost and replaced by maturation from stem cells and by proliferation of mature cells. Labile cells include hematopoietic cells in the bone marrow and the majority of surface epithelia, such as the stratified squamous surfaces of the skin, oral cavity, vagina, and cervix; the cuboidal epithelia of the ducts draining exocrine organs (e.g., salivary glands, pancreas, biliary tract); the columnar epithelium of the gastrointestinal tract, uterus, and fallopian tubes; and the transitional epithelium of the urinary tract. These tissues can readily regenerate after injury as long as the pool of stem cells is preserved.
2- Stable Tissues (quiescent tissues)
Cells of these tissues are quiescent and have only minimal replicative activity in their normal state. However, these cells are capable of proliferating in response to injury or loss of tissue mass. Stable cells constitute:-1-the parenchyma of most solid tissues, such as liver, kidney, and pancreas.
2- endothelial cells.
3- fibroblasts.
4- smooth muscle cells.
the proliferation of these cells is particularly important in wound healing. With the exception of liver, stable tissues have a limited capacity to regenerate after injury.
Permanent Tissues( non dividing tissues )
The cells of these tissues are considered to be terminally differentiated and non proliferative in postnatal life. Ex . The neurons and cardiac muscle cells belong to this category. Thus, injury to brain or heart is irreversible and results in a scar, because neurons and cardiac myocytes do not divide. Limited stem cell replication and differentiation occurs in some areas of the adult brain, and there is some evidence that heart muscle cells may proliferate after myocardial necrosis. Nevertheless, whatever proliferative capacity may exist in these tissues, it is insufficient to produce tissue regeneration after injury. Skeletal muscle is usually classified as a permanent tissue, but satellite cells attached to the endomysial sheath provide some regenerative capacity for this tissue.
Stem Cells
In most continuously dividing tissues the mature cells are terminally differentiated and short-lived. As mature cells die the tissue is replenished by the differentiation of cells generated from stem cells.Stem cells are characterized by :-
1- Self-renewal capacity .
2- Asymmetric replication
means that after each cell division, some progeny enter a differentiation pathway, while others remain undifferentiated, retaining their self-renewal capacity.
3- Bone marrow stem cells have very broad differentiation capabilities, being able to generate fat, cartilage, bone, endothelium, and muscle.
stem cells with the capacity to generate multiple lineages are present in the bone marrow and several other tissues of adult individuals. These cells are called tissue stem cells or adult stem cells.
CONTROL OF CELL PROLIFERATION
Cell proliferation can be triggered by1- chemical mediators, such as growth factors, hormones, and cytokines. hormones and many cytokines are involved in the stimulation or inhibition of cell growth.
2- Signals from the ECM are also important inducers of cell replication.
GROWTH FACTORS
IS protein that expands cell populations by stimulating cell division (usually accompanied by increased cell size) and by promoting cell survival.Most growth factors have pleiotropic effects; that is, in addition to stimulating cellular proliferation, they stimulate migration, differentiation and contractility, and enhance the synthesis of specialized proteins (such as collagen in fibroblasts).
growth factors that involved in repair are produced by :-
1- leukocytes that are recruited to the site of injury or are activated at this site, as part of the inflammatory process.
2- the parenchymal cells or connective tissue cells in response to cell injury or loss.
MECHANISMS OF ACTION OF GROWTH FACTORS
1- stimulate the function of growth control genes , many of which are called protooncogenes because mutations in them lead to uncontroled cell proliferation characteristic of cancer (oncogenesis).
2- stimulate proliferation of some cells and inhibit cycling of other cells.
3- growth factor can have opposite effects on the same cell depending on its concentration. An example of such a growth factor is transforming growth factor-β (TGF-β).
growth factor act as extracellular signals that will bind receptor located on the cell surface or intracellularly . the activation of the receptor will triggers a series of events leading to stimulation or repression of gene expression and cell division .
EXTRACELLULAR MATRIX (ECM) AND CELL-MATRIX INTERACTIONS
Tissue repair depends not only on growth factor activity but also on interactions between cells and ECM components. The ECM is a dynamic, constantly remodeling macromolecular complex synthesized locally, which assembles into a network that surrounds cells.
ECM occurs in two basic forms: interstitial matrix and . basement membrane
Interstitial Matrix
This is present in the spaces between cells in connective tissue, and between epithelium and supportive vascular and smooth muscle structures; it is synthesized by mesenchymal cells (e.g., fibroblasts) . Its major constituents are fibrillar and nonfibrillar collagens, as well as fibronectin, elastin, proteoglycans, hyaluronate, and other elements.Basement membrane
The basement membrane lies beneath the epithelium and is synthesized by overlying epithelium and underlying mesenchymal cells; it tends to form a platelike mesh. Its major constituents are amorphous non fibrillar type IV collagen and laminin.
Roles of the Extracellular Matrix
The ECM is much more than a space filler around cells. Its various functions include:1- Mechanical support for cell anchorage and cell migration.
2- Control of cell growth. ECM components can regulate cell proliferation by signaling through cellular receptors .
3- Maintenance of cell differentiation. The type of ECM proteins can affect the degree of differentiation of the cells in the tissue.
4- Scaffolding for tissue renewal. The maintenance of normal tissue structure requires a basement membrane or stromal scaffold. Disruption of these structures leads to collagen deposition and scar formation.
5-Establishment of tissue microenvironments. Basement membrane acts as a boundary between epithelium and underlying connective tissue .
6- Storage of regulatory molecules. For example, growth factors like FGF .This allows the rapid deployment of growth factors after local injury, or during regeneration.
Components of the Extracellular Matrix
There are three basic components of ECM:
(1) fibrous structural proteins such as collagens and elastins .
(2) water-hydrated gels such as proteoglycans and hyaluronan, .
(3) adhesive glycoproteins ( fibronectin) that connect the matrix elements to one another and to cells.
cutaneous wound healing healing process in general has three main phases:
(1) inflammation,(2) formation of granulation tissue,
(3) ECM deposition and remodeling.
Healing of any wound can occur by one of two types of repair :
1- healing by first intention (primary union ).
2- healing by second intention (secondary union ).
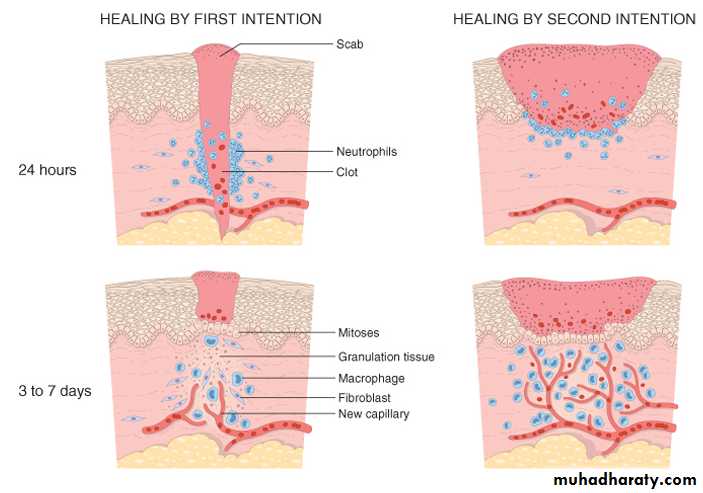
1- healing by first intention (primary union ).
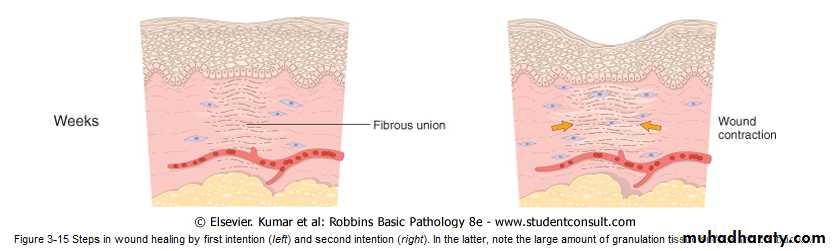
is the healing of a clean, uninfected surgical incision approximated by surgical sutures .This is referred to as primary union, or healing by first intention. The incision causes only focal disruption of epithelial basement membrane continuity and death of a relatively few epithelial and connective tissue cells. As a result, epithelial regeneration predominates over fibrosis. A small scar is formed, but there is minimal wound contraction.2- Healing by Second Intention
When cell or tissue loss is more extensive, such as in large wounds, abscess formation, and ulceration, the repair process is more complex, as is also the case after infarction in parenchymal organs. In second-intention healing, also known as healing by secondary union the inflammatory reaction is more intense, there is abundant development of granulation tissue, and the wound contracts by the action of myofibroblasts. This is followed by accumulation of ECM and formation of a large scar.wound contraction
Secondary healing involves wound contraction. Within 6 weeks, for example, large skin wound may be reduced from the original size, largely by contraction. This process is due to the presence of myofibroblasts, which are modified fibroblasts exhibiting many of the ultrastructural and functional features of contractile smooth muscle cells.
REPAIR BY CONNECTIVE TISSUE
if non dividing cells are injured, repair cannot be accomplished by regeneration alone. Under these conditions, repair occurs by replacement of the non regenerated cells with connective tissue, or by a combination of regeneration of some cells and scar formation.Repair begins within 24 hours of injury by the emigration of fibroblasts and the induction of fibroblast and endothelial cell proliferation.
By 3 to 5 days, a specialized type of tissue that is characteristic of healing, called granulation tissue, is apparent.
The term granulation tissue derives from the pink, soft, granular gross appearance, such as that seen beneath the scab of a skin wound.
Its histologic appearance is characterized by
1-proliferation of fibroblasts .
2- new thin-walled, delicate capillaries (angiogenesis), with a loose ECM.
Granulation tissue then progressively accumulates connective tissue matrix, eventually resulting in the formation of a scar , which may remodel over time.
Repair by connective tissue deposition consists of four sequential processes:
1-Formation of new blood vessels (angiogenesis).2- Migration and proliferation of fibroblasts and
3-Deposition of ECM (scar formation).
4- Maturation and reorganization of the fibrous tissue (remodeling).
1-Formation of new blood vessels (angiogenesis)
angiogenesis, or neovascularization, in which preexisting vessels send out capillary sprouts to produce new vessels. endothelial precursor cells may migrate from the bone marrow to areas of injury and participate in angiogenesis at these sites . Several growth factors induce angiogenesis but most important are vascular endothelial growth factor (VEGF) and fibropalst growth factor (FGF).
2- Migration and proliferation of fibroblasts and scar formation
It occurs in two steps:
(1) migration and proliferation of fibroblasts into the site of injury and (2) deposition of ECM (scar formation) by these cells.
(1) migration and proliferation of fibroblasts into the site of injury
- The recruitment and stimulation of fibroblasts is driven by many growth factors, including platelet-derived growth factor PDGF, basic fibroblast growth factor FGF-2 . One source of these factors is the activated endothelium and inflammatory cells.
- Macrophages, in particular, are important cellular constituents of granulation tissue, and besides clearing extracellular debris and fibrin at the site of injury, they elaborate a host of mediators that induce fibroblast proliferation and ECM production.
- Sites of inflammation are also rich in mast cells , and lymphocytes may also be present. Each of these can contribute directly or indirectly to fibroblast proliferation and activation.
(2) deposition of ECM by these cells (scar formation)
As healing progresses, the number of proliferating fibroblasts and new vessels decreases; however, the fibroblasts progressively assume a more synthetic phenotype, and hence there is increased deposition of ECM. Collagen synthesis, in particular, is critical to the development of strength in a healing wound site. Collagen synthesis by fibroblasts begins early in wound healing (days 3 to 5) and continues for several weeks, depending on the size of the wound. many of the same growth factors that regulate fibroblast proliferation also participate in stimulating ECM synthesis and Scar Formation including PDGF, and FGF . It stimulates the production of collagen causes migration and proliferation of fibroblasts, smooth muscle cells, and macrophages.Cytokines as mediators of inflammation may also function as growth factors and participate in ECM deposition and scar formation. IL-1 and TNF, for example, induce fibroblast proliferation and can have a fibrogenic effect . They are also chemotactic for fibroblasts and stimulate it to synthesis of collagen .
4- Maturation and reorganization of the fibrous tissue ( Remodeling )
The transition from granulation tissue to scar involves changes in the composition of the ECM; even after its synthesis and deposition, scar ECM continues to be remodeled. The outcome of the repair process is, in part, a balance between ECM synthesis and degradation.The degradation of collagens and other ECM components is accomplished by a family of matrix metalloproteinases (MMPs) . MMPs are produced from neutrophil s ,macrophages ,and fibroplast and some epithelial cells. and their synthesis and secretion are regulated by growth factors, cytokines, and other agents .
Factors delay healing
1- Infection is the single most important cause of delay in healing; it prolongs the inflammation phase of the process and potentially increases the local tissue injury.2- Nutrition has profound effects on wound healing; protein deficiency, for example, and particularly vitamin C deficiency, inhibits collagen synthesis and retards healing.
3- Glucocorticoids (steroids) have well-documented anti-inflammatory effects, and their administration may result in poor wound strength due to diminished fibrosis.
4- Mechanical variables such as increased local pressure or torsion may cause wounds to pull apart.
5- Poor perfusion, due either to arteriosclerosis and diabetes or to obstructed venous drainage (e.g. in varicose veins).
6- foreign bodies such as fragments of steel, glass.
Aberrations of cell growth and ECM production
may occur during begins of normal wound healing. For example,
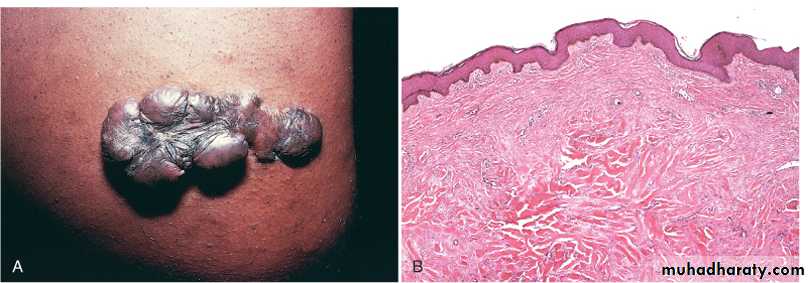
1- keloids the accumulation of exuberant amounts of collagen can give rise to prominent, raised scars. There appears to be a heritable predisposition to keloid formation, and the condition is more common in blacks.
2- exuberant granulation healing wounds may also generate excessive granulation tissue that protrudes above the level of the surrounding skin and hinders re-epithelialization. and restoration of epithelial continuity requires cautery or surgical resection of the granulation tissue .
3- disabling fibrosis associated with chronic inflammatory diseases such as rheumatoid arthritis, pulmonary fibrosis, and cirrhosis have many similarities to those involved in normal wound healing. In these diseases, persistent stimulation of fibrogenesis results from chronic immune reactions that sustain the synthesis and secretion of growth factors, fibrogenic cytokines, and proteases.
Keloid. A, Excess collagen deposition in the skin forming a raised scar known as a keloid. B, Thick connective tissue deposition in the dermis.