HEMOSTASIS AND THROMBOSIS
Normal hemostasis is a regulated processes that maintain blood in a fluid, clot-free state in normal vessels with rapid formation of a localized hemostatic plug at the site of vascular injury.Thrombosis (the pathologic form of hemostasis):- its a formation of blood clot (thrombus) in uninjured vessels or thrombotic occlusion of a vessel after relatively minor injury.
Both hemostasis and thrombosis involve three components:
1- the vascular wall.
2-platelets.
3- the coagulation cascade. .
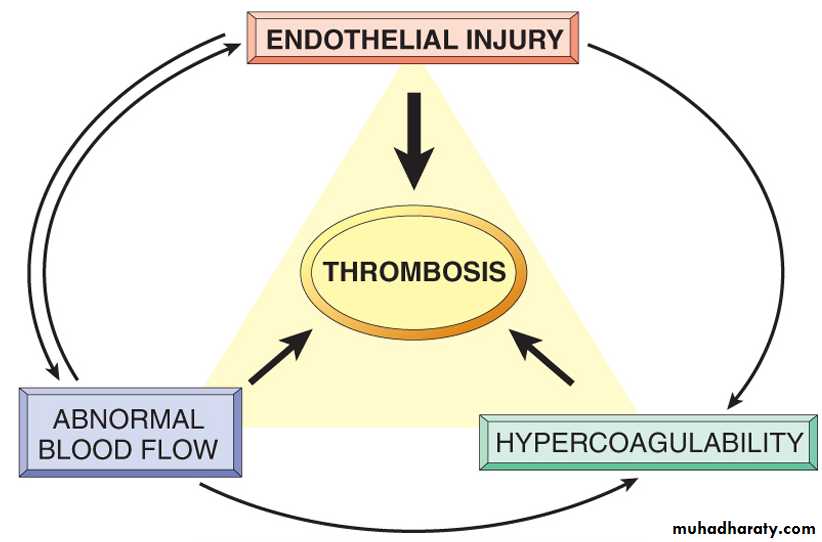
Pathogenesis of thrombosis
There are three primary influences on thrombus formation (called Virchow's triad):(1) endothelial injury.
(2) stasis or turbulence of blood flow.
(3) blood hypercoagulability.
1- Endothelial Injury
It is a dominant influence, since endothelial loss can lead to thrombosis. It is important for thrombus formation occurring in the heart or in the arterial circulation, the normally high flow rates prevent clotting by preventing platelet adhesion or diluting coagulation factors.
physical loss of endothelium leads to exposure of subendothelial ECM, adhesion of platelets, release of coagulation factor that help in thrombus formation .
Virchow's triad in thrombosis. Integrity of endothelium is the most important factor. Injury to endothelial cells can also alter local blood flow and affect coagulability. Abnormal blood flow (stasis or turbulence), in turn, can cause endothelial injury. The factors may act independently or may combine to promote thrombus formation.
2- Alterations in Normal Blood Flow
Turbulence contributes to arterial and cardiac thrombosis by causing endothelial injury or dysfunction, as well as by forming countercurrents and local pockets of stasis;stasis is a major contributor to the development of venous thrombi.
Normal blood flow is laminar, such that platelets flow centrally in the vessel lumen, separated from the endothelium by a slower moving clear zone of plasma.
Stasis and turbulence will :-
1- Disrupt laminar flow and bring platelets into contact with the endothelium.
2- Prevent dilution of activated clotting factors by fresh-flowing blood .
3- Retard the inflow of clotting factor inhibitors and permit the buildup of thrombi.
4- Promote endothelial cell activation, resulting in local thrombosis, leukocyte adhesion, etc .
Causes of turbulence and Stasis
1-Ulcerated atherosclerotic plaques not only expose subendothelial ECM but also cause turbulence.2- Abnormal aortic and arterial dilations, called aneurysms, create local stasis and consequently a fertile site for thrombosis .
3- Acute myocardial infarction results in focally noncontractile myocardium; ventricular remodeling after more remote infarction can lead to aneurysm formation. In both cases cardiac mural thrombi form more easily because of the local blood stasis .
4- Mitral valve stenosis (e.g., after rheumatic heart disease) results in left atrial dilation which is a site of profound stasis and a prime location for development of thrombi.
5- Hyperviscosity syndromes (such as polycythemia) increase resistance to flow and cause small vessel stasis; the deformed red cells in sickle cell anemia cause vascular occlusions, with the resultant stasis also predisposing to thrombosis.
3- Hypercoagulability
its any alteration of the coagulation pathways that predisposes to thrombosis. and it can be divided into primary (genetic) and secondary (acquired) disorders.
1- Primary (inherited) disorders like mutations in the factor V gene and the prothrombin gene.
2- Secondary (acquired) disorders , the pathogenesis of acquired thrombotic diatheses is frequently multifactorial and is therefore more complicated and include
- cardiac failure or trauma stasis or vascular injury may be most important.
- Hypercoagulability is associated with oral contraceptive use and the hyperestrogenic state of pregnancy, probably related to increased hepatic synthesis of coagulation factors and reduced synthesis of antithrombin III.
- In disseminated cancers, release of procoagulant tumor products predisposes to thrombosis.
- The hypercoagulability seen with advancing age has been attributed to increasing platelet aggregation and reduced endothelial PGI2 release.
- Smoking and obesity promote hypercoagulability by unknown mechanisms.
Morphology of thrombus
Thrombi can develop in the cardiovascular system (e.g., in cardiac chambers, on valves, or in arteries, veins, or capillaries). The size and shape of a thrombus depend on the site of origin and the cause. Arterial or cardiac thrombi typically begin at sites of endothelial injury or turbulence; venous thrombi characteristically occur at sites of stasis. Thrombi are attached to the underlying vascular surface; arterial thrombi tend to grow in a retrograde direction from the point of attachment, while venous thrombi extend in the direction of blood flow (thus both tend to propagate toward the heart). The propagating portion of a thrombus tends to be poorly attached and therefore prone to fragmentation, generating an embolusThrombi can have grossly and microscopically apparent laminations called lines of Zahn; these represent pale platelet and fibrin layers alternating with darker erythrocyte-rich layers. These lines distinguish antemortem thrombosis from the bland nonlaminated postmortem clots . Although such lines are typically not as apparent in veins or smaller arteries (thrombi formed in sluggish venous flow usually resemble statically coagulated blood), careful evaluation generally reveals ill-defined laminations.
Types of thrombus
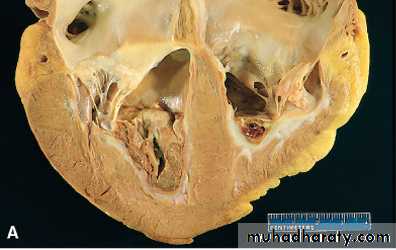
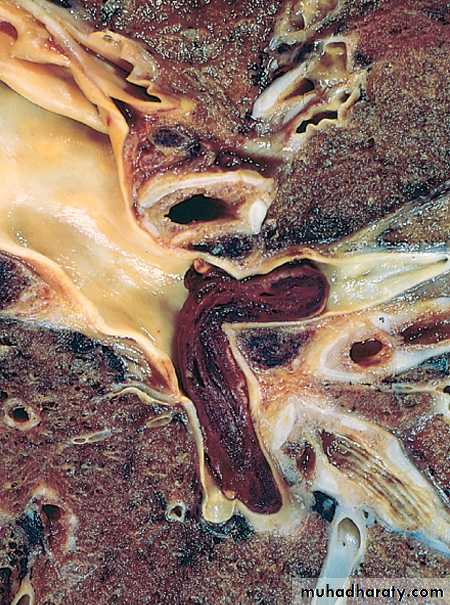
1- mural thrombi Thrombi occurring in heart chambers or in the aortic lumen.Causes
A- Abnormal myocardial contraction (resulting from arrhythmias,or myocardial infarction).
B- endomyocardial injury (caused by myocarditis, catheter trauma) promotes cardiac mural thrombi.
2- Arterial thrombi are frequently occlusive and are produced by platelet and coagulation activation; they are typically a friable meshwork of platelets, fibrin, erythrocytes, and degenerating leukocytes.
Causes
Although arterial thrombi are usually superimposed on an atherosclerotic plaque, other vascular injury (vasculitis, trauma) .
Mural thrombi. A, Thrombus in the left and right ventricular apices, overlying white fibrous scar.
3 - Venous thrombosis (phlebothrombosis)
is almost occlusive, and the thrombus can create a long cast of the lumen; venous thrombosis is largely the result of activation of the coagulation cascade, and platelets play a secondary role. Because these thrombi form in the sluggish venous circulation, they also tend to contain more enmeshed erythrocytes and are therefore called red, or stasis, thrombi. The veins of the lower extremities are most commonly affected .4- vegetations. Bacterial or fungal blood-borne infections can cause valve damage, subsequently leading to large thrombotic masses (infective endocarditis) .
Fate of the Thrombus
If a patient survives the initial thrombosis, in the ensuing days or weeks thrombi undergo some combination of the following four events:1-Propagation. Thrombi accumulate additional platelets and fibrin, eventually causing vessel obstruction.
2- Embolization. Thrombi dislodge or fragment and are transported elsewhere in the vasculature.
3- Dissolution. Thrombi are removed by fibrinolytic activity .
4- Organization and recanalization. Thrombi induce inflammation and fibrosis (organization). These can eventually recanalize (re-establishing some degree of flow), or they can be incorporated into a thickened vessel wall.
Clinical significance
Thrombi are significant because1-cause obstruction of arteries and veins .
2- are potential sources of emboli.
Which effect is most important depends on the site of thrombosis.
Venous thrombi can cause congestion and edema in vascular beds distal to an obstruction, but they are most worrisome for their capacity to embolize to the lungs and cause death.
Arterial thrombi can embolize and even cause downstream tissue infarction , their role in vascular obstruction at critical sites (e.g., coronary and cerebral vessels) is much more significant clinically.
EMBOLISM
is a detached intravascular solid, liquid, or gaseous mass that is carried by the blood to a site distant from its point of origin.
Forms of emboli
1- thromboembolism 99% of all emboli represent a dislodged thrombus.
2- fat embolism microscopic fat globules can be found in the circulation after fracture of long bones ( which contain fatty morrow ).
3- air embolism gas bubble within circulation . Air may enter circulation during a chest wall injury . Air bubble can coalesce to form frothy masses sufficiently large to occlude major vessels .
4- atherosclerotic thrombi (cholesterol emboli) consisting atheromatous depris.
5- tumor emboli made up of fragments of a tumor.
6- bone marrow emboli consisting of bits of bone marrow.
emboli lodge in vessels too small to permit further passage, resulting in partial or complete vascular occlusion. The consequences of thromboembolism include ischemic necrosis (infarction) of downstream tissue.
Pulmonary thromboembolism
venous emboli originate from deep leg vein thrombi above the level of the knee such as femoral or iliac veins . These emboli are carried through progressively larger channels and pass through the right side of the heart before entering the pulmonary arterial circulation . Depending on the size of the embolus, it may occlude the main pulmonary artery, impact across the bifurcation (saddle embolus), or pass out into the smaller, branching arterioles.Embolus derived from a lower extremity deep venous thrombosis and now impacted in a pulmonary artery branch
Systemic Thromboembolism
Systemic thromboembolism refers to emboli in the arterial circulation. Most arise from intracardiac mural thrombi.The major sites for arteriolar embolization are the:-
- lower extremities (75%) .
- the brain (10%),
- the intestines, kidneys, and spleen affected to a lesser extent.
The consequences of embolization in a tissue depend on vulnerability to ischemia, caliber of the occluded vessel, and the collateral blood supply; in general, arterial embolization causes infarction of the affected tissues.
Amniotic Fluid Embolism
caused by entry of amniotic fluid (and its contents) into the maternal circulation via a tear in the placental membranes and rupture of uterine veins. Characterized by
disseminated intravascular coagulation (DIC), due to release of thrombogenic substances from amniotic fluid.
there is marked pulmonary edema and diffuse alveolar damage ,with the pulmonary microcirculation containing squamous cells shed from fetal skin, lanugo hair, fat from vernix caseosa, and mucin derived from the fetal respiratory or gastrointestinal tracts. Systemic fibrin thrombi indicate the onset of Disseminated Intravascular Coagulation (DIC).
INFARCTION
An infarct is an area of ischemic necrosis caused by occlusion of either the arterial supply or the venous drainage in a particular tissue. Nearly 99% of all infarcts result from thrombotic or embolic events, and almost all result from arterial occlusion.Morphology
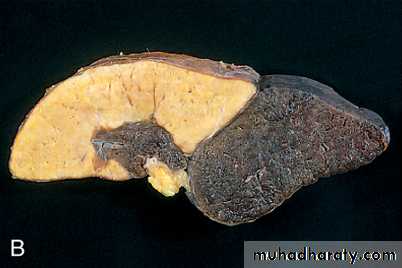
Infarcts are classified on the basis of their color (reflecting the amount of hemorrhage) and the presence or absence of microbial infection. Therefore, infarcts may be either red (hemorrhagic) or white (anemic) and may be either septic or bland( non septic ).
Red infarcts occur
(1) with venous occlusions (such as in ovarian torsion).(2) in loose tissues (such as lung) that allow blood to collect in the infarcted zone.
(3) in tissues with dual circulations such as lung and small intestine, permitting flow of blood from an unobstructed parallel supply into a necrotic area (such perfusion not being sufficient to save the ischemic tissues).
(4) in tissues that were previously congested because of sluggish venous outflow.
(5) when flow is re-established to a site of previous arterial occlusion and necrosis (e.g., fragmentation of an occlusive embolus ).
White infarcts
occur with arterial occlusions or in solid organs (such as heart, spleen, and kidney), where the solidity of the tissue limits the amount of hemorrhage that can seep into the area of ischemic necrosis from adjoining capillary beds .
Septic infarctions occur when bacterial vegetations from a heart valve embolize or when microbes seed an area of necrotic tissue. In these cases the infarct is converted into an abscess, with a correspondingly greater inflammatory response .
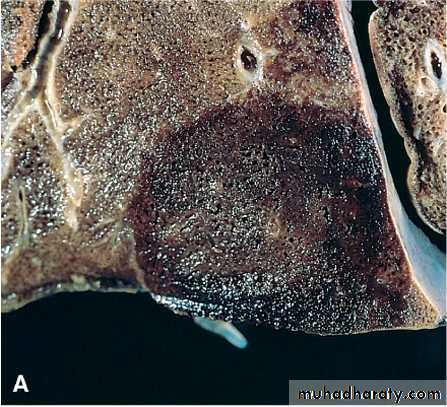
Red and white infarcts. A, Hemorrhagic, roughly wedge-shaped pulmonary infarct (red infarct). B, Sharply demarcated pale infarct in the spleen (white infarct).
Factors That Influence Development of an Infarct
Vascular occlusion can have no or minimal effect, or can cause death of a tissue or even the individual. The major determinants of the eventual outcome include1- Nature of the Vascular Supply The presence of an alternative blood supply is the most important determinant of whether occlusion of a vessel will cause damage. For example,, lungs have a dual pulmonary and bronchial artery blood supply; thus, obstruction of small pulmonary arterioles does not cause infarction in an otherwise healthy individual with an intact bronchial circulation. Similarly, the liver, with its dual hepatic artery and portal vein circulation, and the hand and forearm, with their dual radial and ulnar arterial supply, are all relatively resistant to infarction. In contrast, renal and splenic circulations are end-arterial, and obstruction of such vessels generally causes infarction.
2- Rate of Development of Occlusion Slowly developing occlusions are less likely to cause infarction because they provide time for the development of alternative perfusion pathways. For example, the three major coronary arteries in the heart. If one of the coronaries is slowly occluded (e.g., by atherosclerotic plaque), flow within this collateral circulation may increase sufficiently to prevent infarction, even though the major coronary artery is eventually occluded.
3- Tissue susceptiblity to Hypoxia The susceptibility of a tissue to hypoxia influences the likelihood of infarction. Neurons undergo irreversible damage when deprived of their blood supply for only 3 to 4 minutes. Myocardial cells, though hardier than neurons, are also quite sensitive and die after only 20 to 30 minutes of ischemia. In contrast, fibroblasts within myocardium remain viable after many hours of ischemia.
4- Oxygen Content of Blood The partial pressure of oxygen in blood also determines the outcome of vascular occlusion. Partial flow obstruction of a small vessel in an anemic or cyanotic patient might lead to tissue infarction, whereas it would be without effect under conditions of normal oxygen tension. In this way congestive heart failure, with compromised flow and ventilation, could cause infarction in the setting of an otherwise inconsequential blockage.
Histological appearance
The dominant histologic characteristic of infarction is ischemic coagulative necrosis .In stable or labile tissues, parenchymal regeneration can occur at the periphery. However, most infarcts are ultimately replaced by scar . The brain is an exception to these generalizations; ischemic tissue injury in the central nervous system results in liquefactive necrosis .Shock
Shock is the final step for a number of potentially lethal clinical events, including severe hemorrhage, extensive trauma or burns , large myocardial infarction, massive pulmonary embolism, and microbial sepsis.Shock gives rise to systemic hypoperfusion ; it can be caused either by reduced cardiac output or by reduced effective circulating blood volume . The end results are hypotension , impaired tissue perfusion, and cellular hypoxia. Although the hypoxic and metabolic effects of hypoperfusion initially cause only reversible cellular injury, persistence of shock eventually causes irreversible tissue injury and can culminate in the death of the patient.
Types of shock
1-Cardiogenic shock results from failure of the cardiac pump. This may be caused by myocardial infarction , ventricular arrhythmias, extrinsic compression to the heart , or outflow obstruction (e.g., pulmonary embolism).2- Hypovolemic shock results from loss of blood or plasma volume. This may be caused by hemorrhage, fluid loss from severe burns, or trauma.
3- Septic shock is caused by microbial infection. Most commonly this occurs in gram-negative infections (endotoxic shock), but it can also occur with gram-positive and fungal infections.
4- neurogenic shock may occur in the setting of an anesthetic accident or a spinal cord injury , as a result of loss of vascular tone and peripheral pooling of blood.
5- Anaphylactic shock represents systemic vasodilation and increased vascular permeability caused by an immunoglobulin E hypersensitivity reaction.
Morphology
Shock will induced cellular and tissue necrosis by hypoxic injury or combination of decrease blood flow and microvascular thrombosis. Since shock is characterized by failure of many organ systems, the cellular changes may appear in any tissue. Nevertheless, they are particularly evident in the brain, heart, kidneys, adrenal glands, and gastrointestinal tract. Fibrin thrombi may be identified in any tissue, although they are usually most readily visualized in kidney glomeruli.
clinical manifestations
In hypovolemic and cardiogenic shock, the patient presents with hypotension; a weak, rapid pulse; tachypnea; and cool, clammy, cyanotic skin.In septic shock the skin may be warm and flushed as a result of peripheral vasodilation. The prognosis varies with the type of shock and its duration ,age , and general health of pateints.
Thus, 80% to 90% of young, otherwise healthy patients with hypovolemic shock survive with appropriate management, whereas cardiogenic shock associated with extensive myocardial infarction, or gram-negative sepsis carries a mortality rate of 75%, even with appropriate management .