
Immunoglobulins

Learning objectives
1.Define immunoglobulin.
2. Classify immunoglobulins,
3. Learn the important properties of each class of immunoglobulins
4. Study the antibody function of each class of immunoglobulins.
5. Study the structure of immunoglobulins
6. Monoclonal and polyclonal antibodies.

• The B lymphocytes are mainly derived from bone marrow cells. They
are responsible for the synthesis of circulating humoral antibodies
known as immunoglobulins. They are synthesized mainly in
plasma
cells,
which are specialized cells of B cell and secrete
immunoglobulins into the plasma in response to exposure to a
variety of antigens.
• Entry of foreign molecule into body triggers the synthesis of specific
globulin, which selectively combines with foreign molecule and lead
to its inactivation. The foreign molecule is called as antigen where as
globulin produced against it is called as antibody. Even without
infection the normal plasma contains hundreds of different antibody
molecules.

• Definition
• Immunoglobulins are glycoproteins made up of light and heavy
polypeptide chains. They have the same basic structure (Y shape)
consist of four polypeptide chains.

• Classification
• Immunoglobulins (Igs) are divided into five main classes according to their
molecular weight, electrophoretic mobility, ultracentrifugal sedimentation
and other properties.
• Classes :
1. IgG
2. IgA
3. IgM
4. IgD
5. IgE

Immunoglobulins structure
The basic structure of all Igs is similar.
• They are a glycoproteins composed of
two identical light chains
(molecular weight of each around 25 kDa) and
two identical heavy
chains
, each of 50 to 70 kDa in the form of Y shaped. The four chains
are linked by disulfide bonds an individual antibody molecule always
consist of identical H and identical L chains. L chains may be either
Kappa or Lambda but not both . The heavy chains may be one of five
types and are designated by Greek letter : alpha , gamma , delta, mu
and epsilon corresponding to IgA , IgG, IgD ,IgM and IgE respectively.
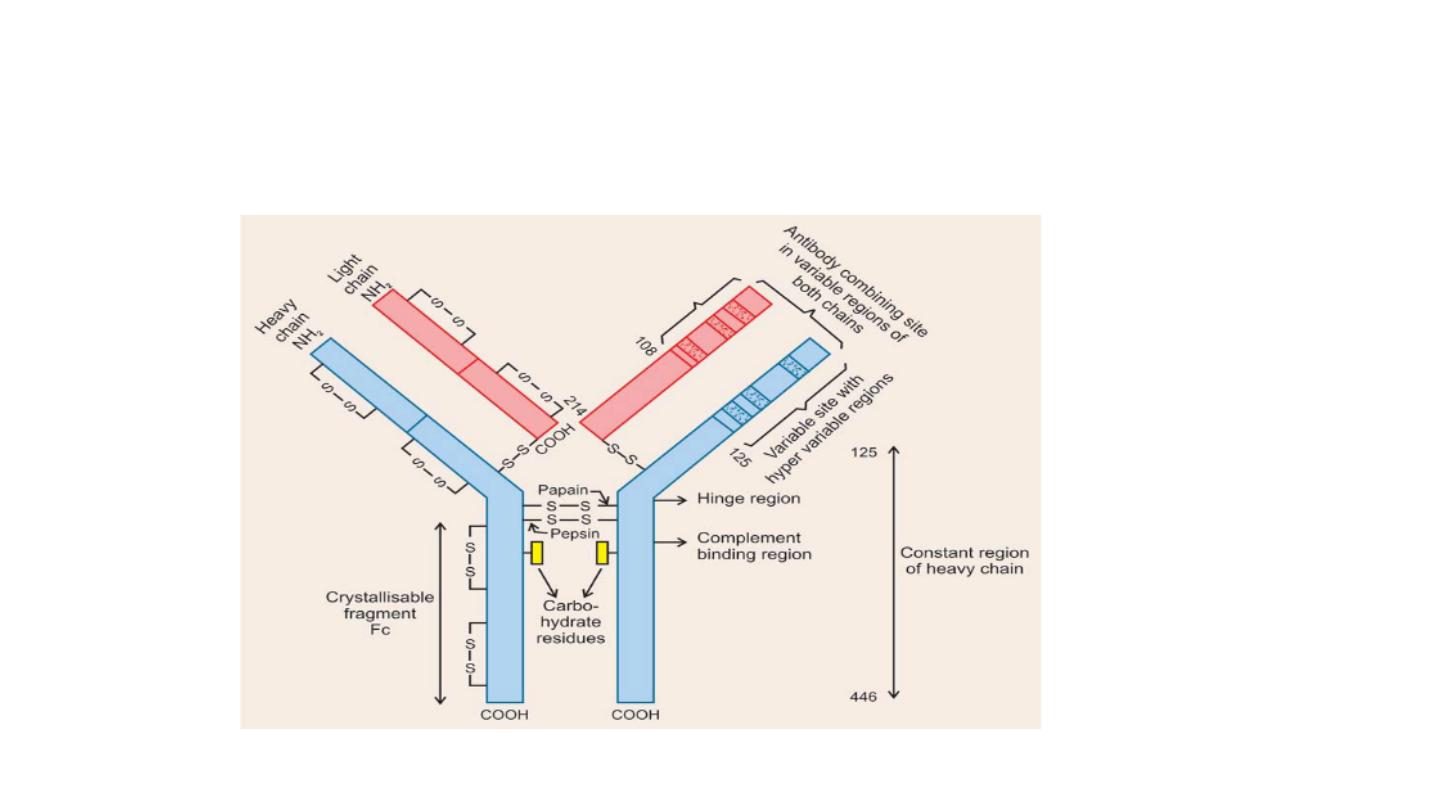

• The light and heavy chains are subdivided onto variable and
constant regions.
• The light chains contain one constant region (CL) and one variable
region (VL). The VL region determines the immunological specificity.
Light chains are attached to the heavy chains by a disulfide bond and
the two heavy chains are held together by disulfide bonds near a
hinge region. The hinge region gives the molecule flexibility needed
in antibody–antigen interactions. The two identical antigen-binding
sites needed in these interactions are formed by the N-terminal part
of one heavy chain and the variable region of one light chain.
• The heavy chain consist of variable region and constant region .
Constant region divided into three region CH1,CH2 and CH3. CH2
region bind to complement while CH3 binding to the cell surface
receptors.

IgG:
• IgG found in highest concentration in blood , so it’s major role in
antibody mediated defense mechanisms.
• MW= 18 0 KDa. They have four subclass IgG1 to IgG4
• It is a monomer.
• Because of its size it can escape from blood vessels more easily. So it
participates in the defense of tissue spaces and body surfaces.
• IgG can opsonize, agglutinate and precipitate antigen.
• Produced mainly in secondary immune response against bacteria and
viruses.
• Is the only antibody that crosses the placenta

IgG has a half-life of approximately 21 days
Function:
• Principal antibody of secondary response
• Crosses placenta and confer fetal immunity
• Opsonizes bacteria, making them easier to phagocytose
• Neutralizes bacterial toxins viruses
• Fixes complement which increases bacterial killing

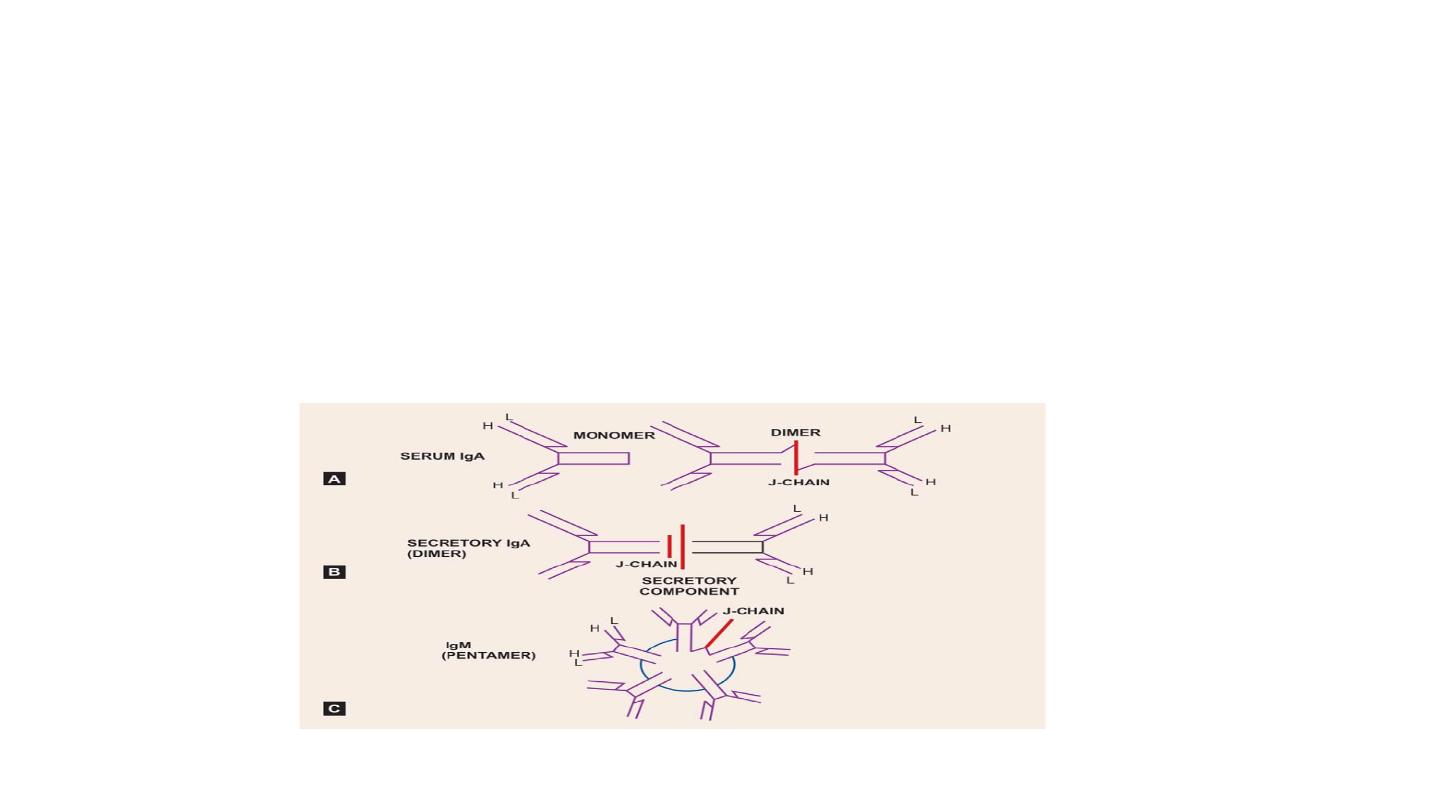
IgA
• IgA is a dimer of 360 KDa, with α-epitopes on its heavy chains.
• There are two form
secretory IgA and serum IgA
• secretory IgA is transported through intestinal epithelial cells or
through hepatocytes, it is bound to a glycoprotein of 71KDa known
as secretory component.
• IgA can’t opsonize antigen, it does not activate complement cascade,
but it can
agglutinate antigen and neutralize viruses
.
• Major role of IgA is to
prevent adherence of antigens to body
surfaces.
• is found in the second highest concentration after IgG

Function:
• Secretory IgA prevents attachment of bacteria and viruses to mucous
membrane
• Does not fix complement.

IgD
•
IgD has a MW of 180 KDa; it has only 2 domains in its
heavy chains because it lacks a CH2. It is a monomer and
resemble IgG structurally Has no known function.
•
IgD along with IgM is the predominant immunoglobulin
on the surface of human B Lymphocytes and it has been
suggested that IgD may be
involved in the differentiation
of these cells
.

Function:
• main function and role yet to be determined
• IgD with antibody activity found towards certain antigens milk
proteins, penicillin, insulin, etc.

IgM
• IgM is formed by five 180 KDa subunits (900 KDa)
• It is a pentamer.
• π epitopes on its heavy chains. - There is additional domain CH4 but
there is no hinge region.
• IgM is the major immunoglobulin
produced in primary immune
response,
but it is also produced in secondary immune responses. It
can
opsonize, neutralize and agglutinate antigens. It can also activate
complement cascade.

Function:
• Produced in the primary response to an antigen
• Does not cross the placenta hence does not give fetal immunity
• Fixes complement , promotes phagocytosis.
• Antigen receptor on the surface of B cells

IgE
• IgE is found in very low concentrations in the serum of
non-parasitized individuals. - IgE has a MW of 200 KDa,
because of the additional domain in the heavy chain near
the hinge region.
•
IgE mediates (type I)
hypersensitivity reactions.
- IgE
largely responsible for immunity to invading parasitic
worms. - IgE has the shortest half-life of all
immunoglobulins (2-3 days) and is readily denatured by
mild heat treatment

• Function:
• allergic and antiparasitic
• Mediates immediate hypersensitivity by causing release of mediators
from mast cells and basophils upon exposure to antigen (allergen or
antibodies).
• Defends against worm infections by causing release of enzymes from
eosinophis.

•
Degradation of Igs by Proteolytic Enzymes
•
Igs are rather insensitive to proteolytic digestion but are
most easily cleaved about midway in the H-chains in an
area between the first and second constant region
domains (CH1 and CH2).
•
(a) Papain: The enzyme Papain splits the molecule on the
N-terminal side of the inter-H chain disulphide bonds into
three fragments of similar size.
• Two “Fab” fragments, which include an entire ‘L’ chain
and the VH and CH-1 domains of a heavy chain, and
• One ‘Fc’ fragment, composed of C-terminal halves of the H-
chains.

• Hinge’ region: The region of the H-chain susceptible to proteolytic
attack is more flexible and exposed to the environment than the
more compact globular domains.

• Antigen Binding Site: Antigen binding activity is associated with the
Fab fragments, or, more specifically with the VH and VL domains.
On the other hand, most of the secondary biologic activities of Igs, e.
g. complement fixation are associated with the ‘Fc’ fragment.
Because there are two Fab regions, IgG molecules bind two
molecules of antigen and are termed divalent. The site on the
antigen to which an antibody binds is termed an antigenic
determinant or epitope.

• Polyclonal and monoclonal antibody.
• Polyclonal antibody: In response to an antigenic challenge the body
produces different types of antibodies against various antigenic
determinants (epitopes) of the antigen. The antibodies thus
produced are called polyclonal antibodies.
• Monoclonal antibody: When one “clone” of antibody producing
cells secrete a particular type of antibody against a particular
antigenic determinant (epitope), it is called monoclonal antibody.

Disorders due to changes in Immunoglobulins :
• Abnormal amounts of certain immunoglobulins may be found in the
plasma in several diseases of human.
1-Multiple myeloma (Bence jones proteins).
The light chains are produced in excess than heavy chains. Because
they are of relatively low molecular weight they pass through
glomerular membrane and appear in the urine .
2- Cryoglobulinemia.
Is serum IgM proteins that precipitates at temperature lower than
body temperature, develop peripheral thrombosis in cold weather.
