
1
Fifth stage
Pediatric
Lec-9
د.ندى العلي
11/4/2016
Diabetes mellitus
The incidence of diabetes in children has increased steadily over the last 20 years and now
affects around 2 per 1000 children by 16 years of age. This is most likely to be a result of
changes in environmental risk factors. There is considerable racial and geographical
variation - Almost all children are insulin-dependent (type 1 diabetes). Type 2 non-insulin-
dependent diabetes due to insulin resistance is starting to occur in childhood as severe
obesity becomes more common..
Classification of diabetes according to aetiology (adapted from American Diabetes
Association,
Type 1. Insulin-dependent
Most childhood diabetes
Type 2. Non-insulin-dependent
Usually older children
obesity-related
positive family history
not prone to ketosis
commoner in some ethnic groups
Type 3. Other specific types
Drugs, e.g. corticosteroids
Pancreatic exocrine insufficiency, e.g. cystic fibrosis
Genetic defects in insulin action
Congenital infection e.g. congenital rubella
Endocrine diseases, e.g. Cushing's syndrome
Genetic/chromosomal syndromes, e.g. Down's and Turner's
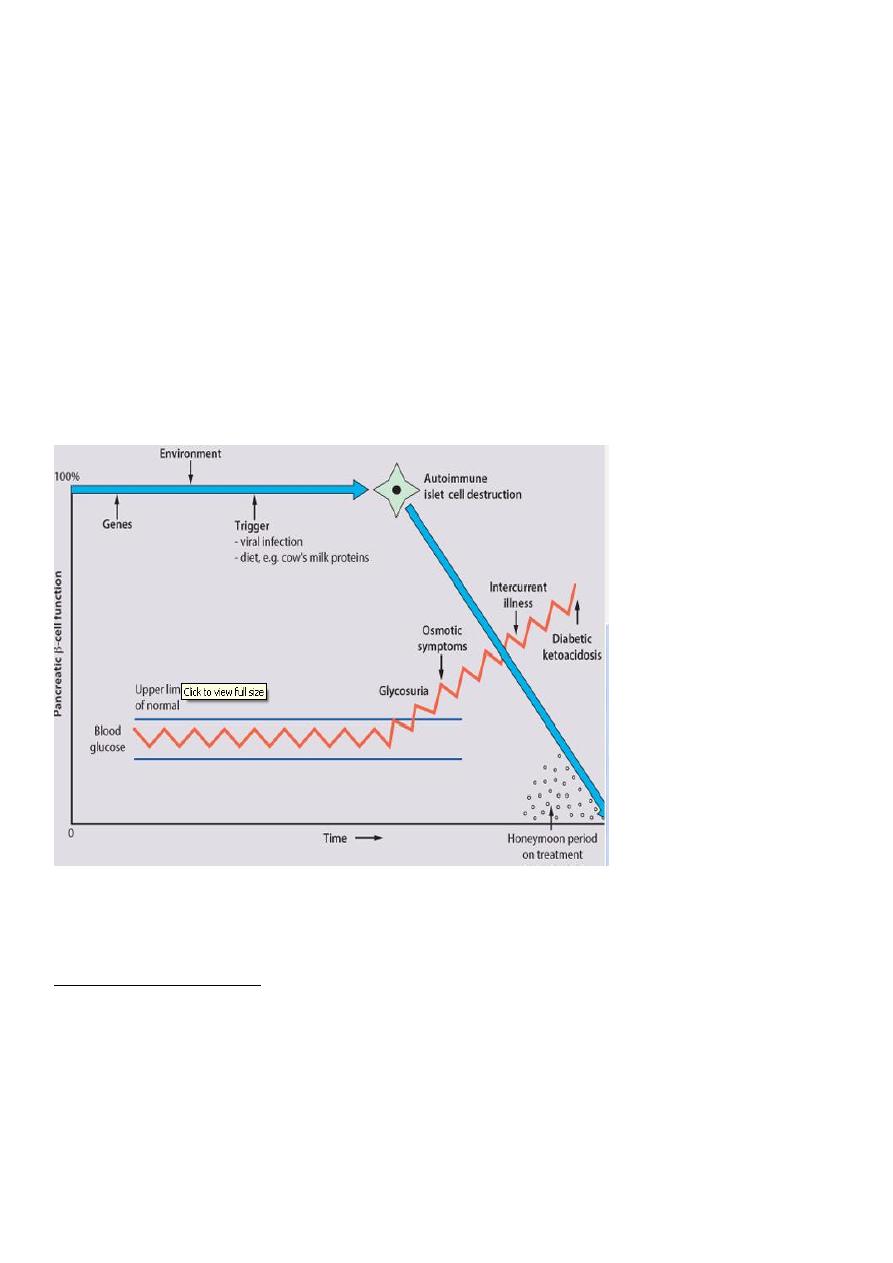
Pathogenesis &Etiology
Both genetic predisposition and environmental precipitants play a role. Inherited
susceptibility is demonstrated by
an identical twin of a diabetic having a 30-50% chance of developing the disease
2
the increased risk of diabetes amongst those who are HLA-DR3 or HLA-DR4 and a reduced
risk with DR2 and DR5
the increased risk of a child developing diabetes if a parent has insulin-dependent diabetes
(1 in 20-40 if the father is affected, 1 in 40-80 if it is the mother
Interaction probably occurs between an environmental trigger and an antigen on the
surface of β-cells of the pancreas.
Triggers which may contribute are viral infections, accounting for the more frequent
presentation in spring and autumn, and diet, possibly cow's milk This results in an
autoimmune process which damages the pancreatic β-cells and leads to an absolute insulin
deficiency. Markers of β-cell destruction include islet cell antibodies and antibodies to
glutamic acid decarboxylase (GAD).
There is an association with other autoimmune disorders such as hypothyroidism
Clinical picture
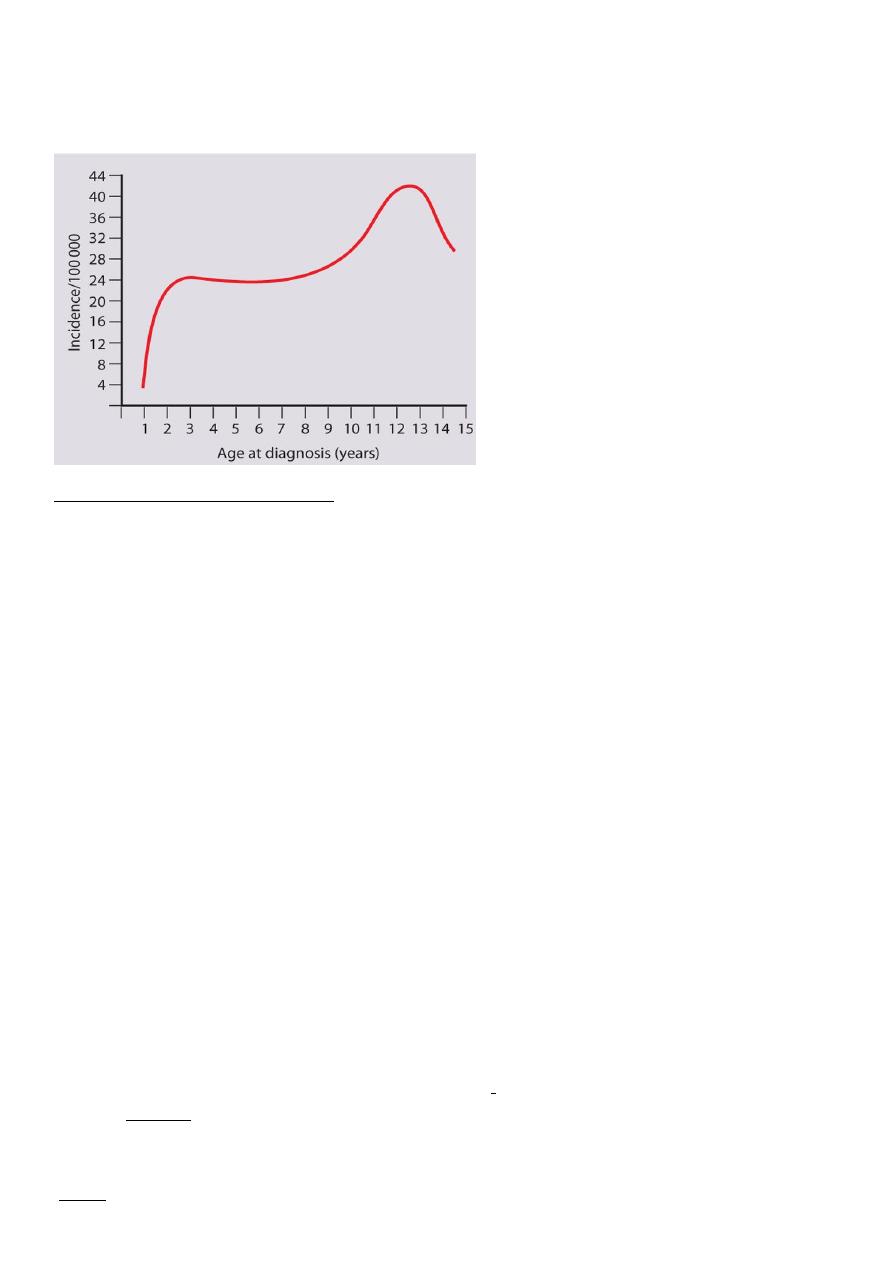
The age at presentation
It is uncommon before the age of 1 year, but the incidence rises steadily during the early
school years Peaks of presentation occur at 5 - 7 years of age and at adolescence
12-13 years of age. In contrast to adults, children usually present with only a few weeks of
polyuria, excessive thirst (polydipsia) and weight loss; young children may also develop
secondary nocturnal enuresis. Most children are diagnosed at this early stage of the illness
3
.Advanced diabetic ketoacidosis has become an uncommon presentation, but requires
urgent recognition and treatment. Diabetic ketoacidosis may be misdiagnosed if the
hyperventilation is mistaken for pneumonia or the abdominal pain for appendicitis .
Symptoms and signs of diabetes
Early
o Most common - the 'classical triad
Excessive drinking polydipsia
Polyuria
Weight loss
o Less common
Enuresis (secondary)
Skin sepsis
Candida and other infections
Late - diabetic ketoacidosis
o Smell of acetone on breath
o Vomiting
o Dehydration
o Abdominal pain
o Hyperventilation due to acidosis (Kussmaul breathing)
o Hypovolaemic shock
o Drowsiness
o Coma
The diagnosis is usually confirmed in asymptomatic child by finding a markedly raised
random blood glucose
>11.1mmol/L or >200 mg/dl
Urine exam: glycosuria and ketonuria

4
A diagnostic glucose
tolerance test is rarely required in children
Type 2 diabetes should be suspected if there is a family history, and in severely obese
children
Initial management of type 1 diabetes
The initial management will depend on the child's clinical condition. Those in advanced
diabetic ketoacidosis require urgent hospital admission and treatment
'Most newly presenting children are alert and able to eat and drink and can be managed
with subcutaneous insulin alone. In some centres, children newly presenting with diabetes
who do not require intravenous therapy are not admitted to hospital but are managed
entirely at home
Insulin
Insulin is made chemically identical to human insulin by recombinant DNA technology or by
chemical modification of pork insulin. All insulin that is used in the UK in children is human
and in concentrations of 100 U/ml (U-100). The types of insulin include :
1.Human insulin analogues. Rapid-acting insulin analogues,
( Humalog and NovoRapid) -
faster onset and shorter duration of action than soluble insulin. There are also very long-
acting insulin analogues, e.g. insulin detemir (Levemir) or glargine .
2.Short-acting soluble insulin. Onset of action (30-60 minutes), peak 2-4 hours, duration up
to 8 hours. Given 15-30 minutes before meals. Examples are Actrapid and Humulin S
3.Intermediate-acting insulin. Onset 1-2 hours, peak 4-12 hours. Isophane insulin is insulin
with protamine,
4.Predetermined preparations of mixed short- and intermediate-acting isophane insulins.
Examples are Mixtard 30/70 and Humulin M3 (contain 30% soluble and 70% isophane
insulin) .
Insulin can be given by injections using a variety of syringe and needle sizes, pen-like
devices
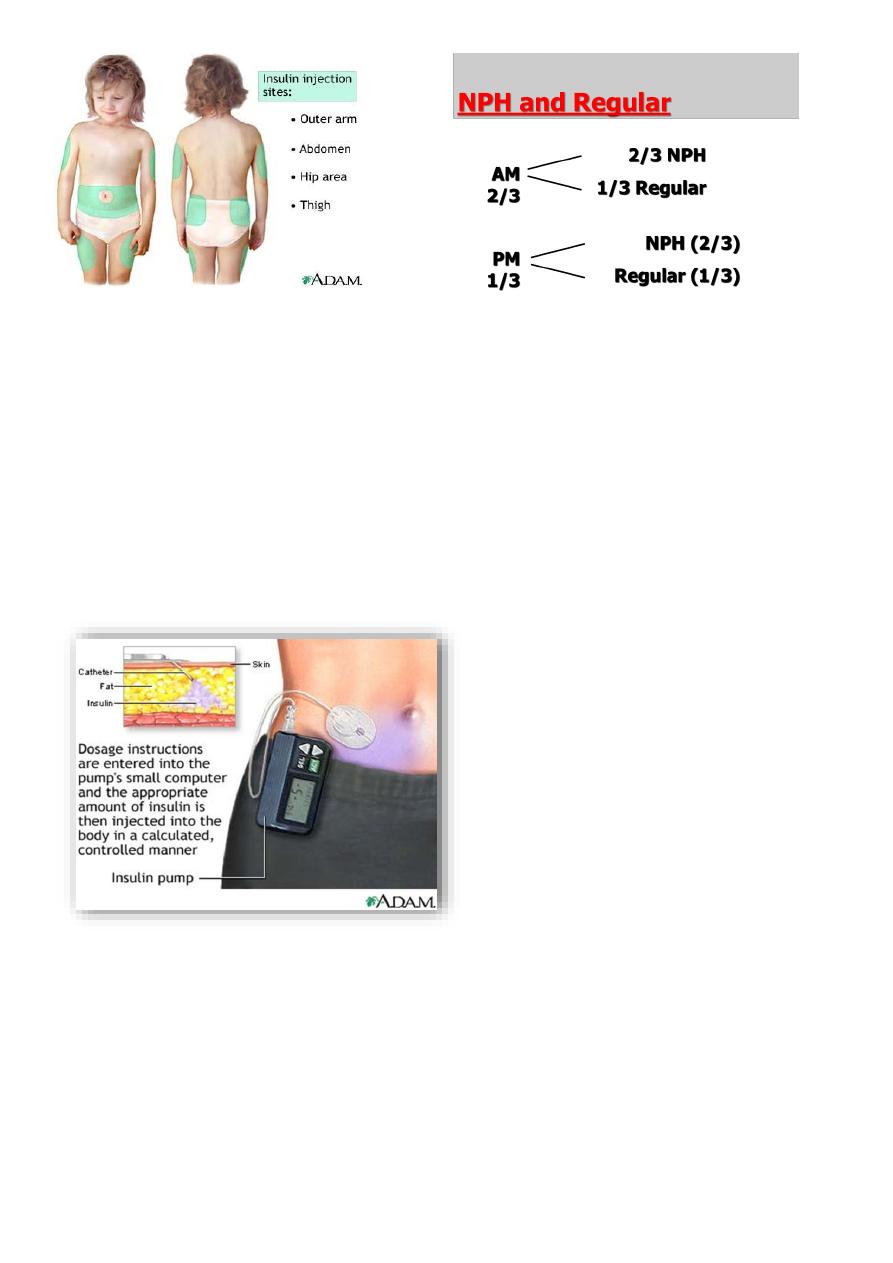
Insulin may be injected into the subcutaneous tissue of the upper arm, the anterior and
lateral aspects of the thigh, the buttocks and the abdomen. Rotation of the injection sites is
essential to prevent lipohypertrophy or, more rarely, lipoatrophy. The skin should be
pinched up and the insulin injected at a 45° angle. Using a long needle or an injection
technique that is 'too vertical' causes a painful, bruised intramuscular injection. Shallow
intra-dermal injections can also cause scarring and should be avoided
5
In young children, insulin is usually given twice a day, before breakfast and evening meals,
as a mixture of short-acting (approximately 30% and medium- or long-acting insulin
pproximately 70%)
In general, about two-thirds of the daily dose is given before breakfast and one-third
before the evening meal.
Continuous subcutaneous insulin infusion (CSII) delivered by a micro-processor controlled
pump can approximate insulin delivery to physiological requirements but requires the use
of an indwelling plastic needle and multiple tests to achieve optimum control and so is not
suitable for all patients .
Shortly after presentation, when some pancreatic function is preserved, insulin
requirements often become minimal, the so-called 'honeymoon period'. Requirements
subsequently increase to 0.5-1 or even up to 2 units/kg per day during puberty .
The diet and insulin regimen need to be matched
(
The aim is to optimise metabolic control whilst maintaining normal growth. On the
standard twice-daily regimen, food intake is divided into three main meals with snacks
between meals and before going to bed

6
A healthy diet is recommended, with a high complex carbohydrate and relatively low fat
content (<30% of total calories). The diet should be high in fiber, which will provide a
sustained release of glucose
rather than refined carbohydrate, which causes rapid swings in glucose levels .
Blood glucose monitoring
Regular blood glucose measurements: (when a low or high level is suspected) are required
to adjust the insulin regimen and learn how changes in lifestyle, food and exercise affect
control.
A record should be kept in a diary or transferred from the memory of the blood glucose
meter.
The aim is to maintain blood glucose as near to normal (4-6 mmol/L) as possible. In
practice, in order also to avoid hypoglycaemic episodes, this means levels of 4-10 mmol/L in
children80-180mg/dl
Urine glucose testing: may rarely be substituted in the very young. Urine or blood ketone
testing is mandatory during infections or when control is poor to avoid severe ketoacidosis .
The measurement of glycosylated haemoglobin (HbA1\C)
is particularly helpful as a guide of overall control over the previous 6 weeks and should be
checked regularly. The level is directly related to the risk of later complications, but may be
misleading if the red blood cell lifespan is reduced, such as in sickle cell trait or if the HbA
molecule is abnormal, as in thalassaemia. A level of less than 7% is an often stated but
rarely achievable target
Diabetic ketoacidosis
Clinical picture:
Keotacidosis is responsible for the initial presentation of IDDM in up to 25% of children.
early manifestations are mild and include vomiting, polyuria, and dehydration.
More severe cases include Kussmaul respirations, odor of acetone on the breath.
abdominal pain or rigidity may be present and mimic acute appendicitis or pancreatitis.
cerebral obtundation and coma ultimately ensue.

7
Essential early investigations:
Blood glucose(11.1mmol/L) or (>300mg/dl)
Urea and electrolytes, creatinine (dehydration)
Blood gas analysis (severe metabolic acidosis)
Urinary glucose and ketones (both are present
(
Evidence of a precipitating cause, e.g. infection (blood and urine cultures performed(
Cardiac monitor for T-wave changes of hypokalaemia
Treatment
Treatment is divided into 3 phases
treatment of ketoacidosis
transition period
continuing phase and guidance
Goals of treatment of DKA
intravascular volume expansion
correction of deficits in fluids, electrolytes, and acid-base status
initiation of insulin therapy to correct catabolism, acidosis
Intravascular volume expansion
dehydration is most commonly in the order of 10%
initial hydrating fluid should be isotonic saline
this alone will often slightly lower the blood glucose
rarely is more than 20 cc/kg fluid required to restore hemodynamics
Treatment of electrolyte abnormalities
serum K+ is often elevated, though total body K+ is depleted
K+ is started early as resolution of acidosis and the administration of insulin will cause a
decrease in serum K+
Insulin Therapy
continuous infusion of low-dose insulin IV (~ 0.1 U/kg/hr) is effective, simple, and
physiologically sound or 0.5-1u/kg then o.2-o.4 every 6hr
goal is to slowly decrease serum glucose, frequent laboratory and blood gas analyses are
obtained to ensure ongoing resolution of metabolic acidosis
8
Hypoglycaemia in diabetes
Hypoglycemic Reactions (Insulin Shock)
symptoms and signs include
pallor, sweating, apprehension,trembling,tachycardia, hunger, drowsiness, mental
confusion, seizures and coma
management includes administration (if conscious) of carbohydrate-containing snack or
drink glucagon 0.5 mg is administered to an unconscious or vomiting child
Severe hypoglycemia can usually be predicted (or explained in retrospect - missed meal,
heavy exercise). The aim is anticipation and prevention. Hypoglycemia in an unconscious
child brought to hospital is treated with glucose given intravenously
Treating a 'hypo' at an early stage requires the administration of easily absorbed glucose in
the form of gulcose tablets or a sugary drink. Children should always have easy access to
their hypo remedy, although young children quickly learn to complain of hypo symptoms in
order to leave class or obtain a sweet drink! Oral glucose gels (e.g. Hypostop) are easily and
quickly absorbed from the buccal mucosa and so are helpful if the child is unwilling or
unable to cooperate to eat. It can be administered by teachers or other helpers
Monitoring
Short term
Home blood glucose monitoring: technique
and methods of analysis and interpretation
Urine testing
Long term
Glycosylated hemoglobin levels
Clinical complications
MONITORING COMPLICATIONS
Careful monitoring of blood pressures is an important part of monitoring procedures.
Monitoring quantitative urinary albumin excretion also appears to be significant because
microalbuminuria exhibits a relationship to the development of significant nephropathy.
Yearly monitoring of thyroid function
blood cholesterol/triglyceride levels is recommended. Complete ophthalmological
examination
and urinalysis must become regular components of ongoing management, beginning
approximately 5 years after the clinical onset of type I or type II diabetes.
